Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii
- PMID: 23152826
- PMCID: PMC3494687
- DOI: 10.1371/journal.pone.0048949
Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii
Abstract
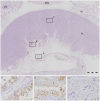
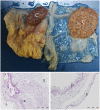
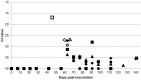
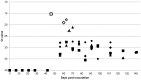
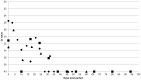
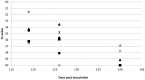
Coxiella burnetii is an intracellular bacterial pathogen that causes Q fever. Infected pregnant goats are a major source of human infection. However, the tissue dissemination and excretion pathway of the pathogen in goats are still poorly understood. To better understand Q fever pathogenesis, we inoculated groups of pregnant goats via the intranasal route with a recent Dutch outbreak C. burnetii isolate. Tissue dissemination and excretion of the pathogen were followed for up to 95 days after parturition. Goats were successfully infected via the intranasal route. PCR and immunohistochemistry showed strong tropism of C. burnetii towards the placenta at two to four weeks after inoculation. Bacterial replication seemed to occur predominantly in the trophoblasts of the placenta and not in other organs of goats and kids. The amount of C. burnetii DNA in the organs of goats and kids increased towards parturition. After parturition it decreased to undetectable levels: after 81 days post-parturition in goats and after 28 days post-parturition in kids. Infected goats gave birth to live or dead kids. High numbers of C. burnetii were excreted during abortion, but also during parturition of liveborn kids. C. burnetii was not detected in faeces or vaginal mucus before parturition. Our results are the first to demonstrate that pregnant goats can be infected via the intranasal route. C. burnetii has a strong tropism for the trophoblasts of the placenta and is not excreted before parturition; pathogen excretion occurs during birth of dead as well as healthy animals. Besides abortions, normal deliveries in C. burnetii-infected goats should be considered as a major zoonotic risk for Q fever in humans.
Conflict of interest statement
Figures

References
-
- Babudieri B (1959) Q fever: a zoonosis. Adv Vet Sci: 81–154.
-
- Oyston PC, Davies C (2011) Q fever: the neglected biothreat agent. J Med Microbiol 60: 9–21. - PubMed
-
- Raoult D, Marrie TJ, Mege JL (2005) Natural history and pathophysiology of Q fever. Lancet Infect Dis 5: 219–226. - PubMed
-
- Angelakis E, Raoult D (2010) Q Fever. Vet Microbiol 140: 297–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

