IL-12p40 deficiency leads to uncontrolled Trypanosoma cruzi dissemination in the spinal cord resulting in neuronal death and motor dysfunction
- PMID: 23152844
- PMCID: PMC3495776
- DOI: 10.1371/journal.pone.0049022
IL-12p40 deficiency leads to uncontrolled Trypanosoma cruzi dissemination in the spinal cord resulting in neuronal death and motor dysfunction
Abstract
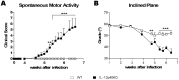
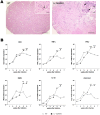
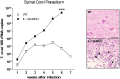
Chagas' disease is a protozoosis caused by Trypanosoma cruzi that frequently shows severe chronic clinical complications of the heart or digestive system. Neurological disorders due to T. cruzi infection are also described in children and immunosuppressed hosts. We have previously reported that IL-12p40 knockout (KO) mice infected with the T. cruzi strain Sylvio X10/4 develop spinal cord neurodegenerative disease. Here, we further characterized neuropathology, parasite burden and inflammatory component associated to the fatal neurological disorder occurring in this mouse model. Forelimb paralysis in infected IL-12p40KO mice was associated with 60% (p<0.05) decrease in spinal cord neuronal density, glutamate accumulation (153%, p<0.05) and strong demyelization in lesion areas, mostly in those showing heavy protein nitrosylation, all denoting a neurotoxic degenerative profile. Quantification of T. cruzi 18S rRNA showed that parasite burden was controlled in the spinal cord of WT mice, decreasing from the fifth week after infection, but progressive parasite dissemination was observed in IL-12p40KO cords concurrent with significant accumulation of the astrocytic marker GFAP (317.0%, p<0.01) and 8-fold increase in macrophages/microglia (p<0.01), 36.3% (p<0.01) of which were infected. Similarly, mRNA levels for CD3, TNF-α, IFN-γ, iNOS, IL-10 and arginase I declined in WT spinal cords about the fourth or fifth week after infection, but kept increasing in IL-12p40KO mice. Interestingly, compared to WT tissue, lower mRNA levels for IFN-γ were observed in the IL-12p40KO spinal cords up to the fourth week of infection. Together the data suggest that impairments of parasite clearance mechanisms in IL-12p40KO mice elicit prolonged spinal cord inflammation that in turn leads to irreversible neurodegenerative lesions.
Conflict of interest statement
Figures


References
-
- Morel CM, Lazdins J (2003) Chagas disease. Nat Rev Microbiol 1: 14–15. - PubMed
-
- Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. - PubMed
-
- Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, et al. (2010) The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev Mol Med 12: e29. - PubMed
-
- Benitez-Hernandez I, Mendez-Enriquez E, Ostoa P, Fortoul T, Ramirez JA, et al. (2010) Proteolytic cleavage of chemokines by Trypanosoma cruzi’s cruzipain inhibits chemokine functions by promoting the generation of antagonists. Immunobiology 215: 413–426. - PubMed
-
- Erdmann H, Steeg C, Koch-Nolte F, Fleischer B, Jacobs T (2009) Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E). Cell Microbiol 11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

