A 3.7 Mb deletion encompassing ZEB2 causes a novel polled and multisystemic syndrome in the progeny of a somatic mosaic bull
- PMID: 23152852
- PMCID: PMC3494662
- DOI: 10.1371/journal.pone.0049084
A 3.7 Mb deletion encompassing ZEB2 causes a novel polled and multisystemic syndrome in the progeny of a somatic mosaic bull
Abstract
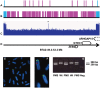
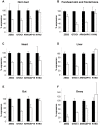
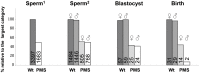
Polled and Multisystemic Syndrome (PMS) is a novel developmental disorder occurring in the progeny of a single bull. Its clinical spectrum includes polledness (complete agenesis of horns), facial dysmorphism, growth delay, chronic diarrhea, premature ovarian failure, and variable neurological and cardiac anomalies. PMS is also characterized by a deviation of the sex-ratio, suggesting male lethality during pregnancy. Using Mendelian error mapping and whole-genome sequencing, we identified a 3.7 Mb deletion on the paternal bovine chromosome 2 encompassing ARHGAP15, GTDC1 and ZEB2 genes. We then produced control and affected 90-day old fetuses to characterize this syndrome by histological and expression analyses. Compared to wild type individuals, affected animals showed a decreased expression of the three deleted genes. Based on a comparison with human Mowat-Wilson syndrome, we suggest that deletion of ZEB2, is responsible for most of the effects of the mutation. Finally sperm-FISH, embryo genotyping and analysis of reproduction records confirmed somatic mosaicism in the founder bull and male-specific lethality during the first third of gestation. In conclusion, we identified a novel locus involved in bovid horn ontogenesis and suggest that epithelial-to-mesenchymal transition plays a critical role in horn bud differentiation. We also provide new insights into the pathogenicity of ZEB2 loss of heterozygosity in bovine and humans and describe the first case of male-specific lethality associated with an autosomal locus in a non-murine mammalian species. This result sets PMS as a unique model to study sex-specific gene expression/regulation.
Conflict of interest statement
Figures


References
-
- Geist V (1966) The Evolution of Horn-Like Organs. Behaviour 27: 175–214.
-
- Janis C (1982) Evolution of horns in ungulates: ecology and paleoecology. Biol Rev 57: 261–318.
-
- Bubenik GA, Bubenik AB (1990) Horns, Pronghorns, and Antlers. Evolution, Morphology, Physiology, and Social Significance. New York: Springer-Verlag. - PubMed
-
- Mitchell G, Skinner JD (2003) On the origin, evolution and phylogeny of giraffes Giraffa camelopardalis. T Roy Soc S Afr 58: 51–73.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

