A Baculovirus immediate-early gene, ie1, promoter drives efficient expression of a transgene in both Drosophila melanogaster and Bombyx mori
- PMID: 23152896
- PMCID: PMC3496687
- DOI: 10.1371/journal.pone.0049323
A Baculovirus immediate-early gene, ie1, promoter drives efficient expression of a transgene in both Drosophila melanogaster and Bombyx mori
Abstract
Many promoters have been used to drive expression of heterologous transgenes in insects. One major obstacle in the study of non-model insects is the dearth of useful promoters for analysis of gene function. Here, we investigated whether the promoter of the immediate-early gene, ie1, from the Bombyx mori nucleopolyhedrovirus (BmNPV) could be used to drive efficient transgene expression in a wide variety of insects. We used a piggyBac-based vector with a 3xP3-DsRed transformation marker to generate a reporter construct; this construct was used to determine the expression patterns driven by the BmNPV ie1 promoter; we performed a detailed investigation of the promoter in transgene expression pattern in Drosophila melanogaster and in B. mori. Drosophila and Bombyx belong to different insect orders (Diptera and Lepidoptera, respectively); however, and to our surprise, ie1 promoter-driven expression was evident in several tissues (e.g., prothoracic gland, midgut, and tracheole) in both insects. Furthermore, in both species, the ie1 promoter drove expression of the reporter gene from a relatively early embryonic stage, and strong ubiquitous ie1 promoter-driven expression continued throughout the larval, pupal, and adult stages by surface observation. Therefore, we suggest that the ie1 promoter can be used as an efficient expression driver in a diverse range of insect species.
Conflict of interest statement
Figures



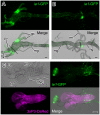

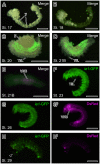



References
-
- Horn C, Schmid BGM, Pogoda FS, Wimmer EA (2002) Fluorescent transformation markers for insect transgenesis. Insect Biochem Mol Biol 32: 1221–1235. - PubMed
-
- Berghammer AJ, Klingler M, Wimmer EA (1999) A universal marker for transgenic insects. Nature 402: 370–371. - PubMed
-
- Thomas JL, Da Rocha M, Besse A, Mauchamp B, Chavancy G (2002) 3×P3-EGFP marker facilitates screening for transgenic silkworm Bombyx mori L. from the embryonic stage onwards. Insect Biochem Mol Biol 32: 247–253. - PubMed
-
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, et al. (2000) Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18: 81–84. - PubMed
-
- Peloquin JJ, Thibault ST, Staten R, Miller TA (2000) Germ-line transformation of pink bollworm (Lepidoptera: Gelechiidae) mediated by the piggyBac transposable element. Insect Mol Biol 9: 323–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

