Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages
- PMID: 23152930
- PMCID: PMC3495862
- DOI: 10.1371/journal.pone.0049741
Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages
Abstract
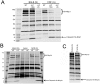
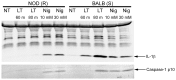
Anthrax lethal factor (LF) is the protease component of anthrax lethal toxin (LT). LT induces pyroptosis in macrophages of certain inbred mouse and rat strains, while macrophages from other inbred strains are resistant to the toxin. In rats, the sensitivity of macrophages to toxin-induced cell death is determined by the presence of an LF cleavage sequence in the inflammasome sensor Nlrp1. LF cleaves rat Nlrp1 of toxin-sensitive macrophages, activating caspase-1 and inducing cell death. Toxin-resistant macrophages, however, express Nlrp1 proteins which do not harbor the LF cleavage site. We report here that mouse Nlrp1b proteins are also cleaved by LF. In contrast to the situation in rats, sensitivity and resistance of Balb/cJ and NOD/LtJ macrophages does not correlate to the susceptibility of their Nlrp1b proteins to cleavage by LF, as both proteins are cleaved. Two LF cleavage sites, at residues 38 and 44, were identified in mouse Nlrp1b. Our results suggest that the resistance of NOD/LtJ macrophages to LT, and the inability of the Nlrp1b protein expressed in these cells to be activated by the toxin are likely due to polymorphisms other than those at the LF cleavage sites.
Conflict of interest statement
Figures


References
-
- Boyden ED, Dietrich WF (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38: 240–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

