Reactivity of diphenylpropynone derivatives toward metal-associated amyloid-β species
- PMID: 23153071
- PMCID: PMC3568435
- DOI: 10.1021/ic302084g
Reactivity of diphenylpropynone derivatives toward metal-associated amyloid-β species
Abstract
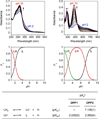
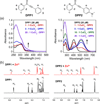
In Alzheimer's disease (AD), metal-associated amyloid-β (metal-Aβ) species have been suggested to be involved in neurotoxicity; however, their role in disease development is still unclear. To elucidate this aspect, chemical reagents have been developed as valuable tools for targeting metal-Aβ species, modulating the interaction between the metal and Aβ, and subsequently altering metal-Aβ reactivity. Herein, we report the design, preparation, characterization, and reactivity of two diphenylpropynone derivatives (DPP1 and DPP2) composed of structural moieties for metal chelation and Aβ interaction (bifunctionality). The interactions of these compounds with metal ions and Aβ species were confirmed by UV-vis, NMR, mass spectrometry, and docking studies. The effects of these bifunctional molecules on the control of in vitro metal-free and metal-induced Aβ aggregation were investigated and monitored by gel electrophoresis and transmission electron microscopy (TEM). Both DPP1 and DPP2 showed reactivity toward metal-Aβ species over metal-free Aβ species to different extents. In particular, DPP2, which contains a dimethylamino group, exhibited greater reactivity with metal-Aβ species than DPP1, suggesting a structure-reactivity relationship. Overall, our studies present a new bifunctional scaffold that could be utilized to develop chemical reagents for investigating metal-Aβ species in AD.
Figures





References
-
- Alzheimer's Association. Alzheimers Dement. 2012;8:131–168. - PubMed
-
- Kepp KP. Chem. Rev. 2012;112:5193–5239. - PubMed
-
- Hardy JA, Higgins GA. Science. 1992;256:184–185. - PubMed
-
- Jakob-Roetne R, Jacobsen H. Angew. Chem. Int. Ed. 2009;48:3030–3059. - PubMed
-
- Rauk A. Chem. Soc. Rev. 2009;38:2698–2715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

