Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth
- PMID: 23153533
- PMCID: PMC3500524
- DOI: 10.1016/j.ccr.2012.09.020
Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth
Abstract
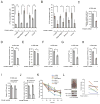
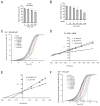
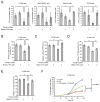
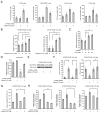
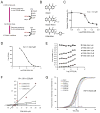
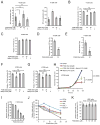
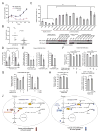
It is unclear how cancer cells coordinate glycolysis and biosynthesis to support rapidly growing tumors. We found that the glycolytic enzyme phosphoglycerate mutase 1 (PGAM1), commonly upregulated in human cancers due to loss of TP53, contributes to biosynthesis regulation in part by controlling intracellular levels of its substrate, 3-phosphoglycerate (3-PG), and product, 2-phosphoglycerate (2-PG). 3-PG binds to and inhibits 6-phosphogluconate dehydrogenase in the oxidative pentose phosphate pathway (PPP), while 2-PG activates 3-phosphoglycerate dehydrogenase to provide feedback control of 3-PG levels. Inhibition of PGAM1 by shRNA or a small molecule inhibitor PGMI-004A results in increased 3-PG and decreased 2-PG levels in cancer cells, leading to significantly decreased glycolysis, PPP flux and biosynthesis, as well as attenuated cell proliferation and tumor growth.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Comment in
-
PGAMgnam style: a glycolytic switch controls biosynthesis.Cancer Cell. 2012 Nov 13;22(5):565-6. doi: 10.1016/j.ccr.2012.10.014. Cancer Cell. 2012. PMID: 23153529
References
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews Cancer. 2011;11:85–95. - PubMed
-
- Chen YY, Ko TP, Chen WH, Lo LP, Lin CH, Wang AH. Conformational changes associated with cofactor/substrate binding of 6-phosphogluconate dehydrogenase from Escherichia coli and Klebsiella pneumoniae: Implications for enzyme mechanism. J Struct Biol. 2010;169:25–35. - PubMed
-
- Corcoran CA, Huang Y, Sheikh MS. The regulation of energy generating metabolic pathways by p53. Cancer biology & therapy. 2006;5:1610–1613. - PubMed
-
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. - PubMed
-
- Engel M, Mazurek S, Eigenbrodt E, Welter C. Phosphoglycerate mutase-derived polypeptide inhibits glycolytic flux and induces cell growth arrest in tumor cell lines. J Biol Chem. 2004;279:35803–35812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 CA164015/CA/NCI NIH HHS/United States
- R01 CA157996/CA/NCI NIH HHS/United States
- R01 GM071440/GM/NIGMS NIH HHS/United States
- CA140515/CA/NCI NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 NS051630/NS/NINDS NIH HHS/United States
- R21 NS067461/NS/NINDS NIH HHS/United States
- K01 HG006699/HG/NHGRI NIH HHS/United States
- T32 GM008602/GM/NIGMS NIH HHS/United States
- CA120272/CA/NCI NIH HHS/United States
- R01 CA140515/CA/NCI NIH HHS/United States
- GM071440/GM/NIGMS NIH HHS/United States
- R01 CA120272/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

