RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal
- PMID: 23154075
- PMCID: PMC3619011
- DOI: 10.1016/j.ijrobp.2012.09.023
RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal
Abstract
Purpose: A multi-institutional phase 2 trial assessed the utility of dose-painted intensity modulated radiation therapy (DP-IMRT) in reducing grade 2+ combined acute gastrointestinal and genitourinary adverse events (AEs) of 5-fluorouracil (5FU) and mitomycin-C (MMC) chemoradiation for anal cancer by at least 15% compared with the conventional radiation/5FU/MMC arm from RTOG 9811.
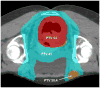
Methods and materials: T2-4N0-3M0 anal cancer patients received 5FU and MMC on days 1 and 29 of DP-IMRT, prescribed per stage: T2N0, 42 Gy elective nodal and 50.4 Gy anal tumor planning target volumes (PTVs) in 28 fractions; T3-4N0-3, 45 Gy elective nodal, 50.4 Gy ≤ 3 cm or 54 Gy >3 cm metastatic nodal and 54 Gy anal tumor PTVs in 30 fractions. The primary endpoint is described above. Planned secondary endpoints assessed all AEs and the investigator's ability to perform DP-IMRT.
Results: Of 63 accrued patients, 52 were evaluable. Tumor stage included 54% II, 25% IIIA, and 21% IIIB. In primary endpoint analysis, 77% experienced grade 2+ gastrointestinal/genitourinary acute AEs (9811 77%). There was, however, a significant reduction in acute grade 2+ hematologic, 73% (9811 85%, P=.032), grade 3+ gastrointestinal, 21% (9811 36%, P=.0082), and grade 3+ dermatologic AEs 23% (9811 49%, P<.0001) with DP-IMRT. On initial pretreatment review, 81% required DP-IMRT replanning, and final review revealed only 3 cases with normal tissue major deviations.
Conclusions: Although the primary endpoint was not met, DP-IMRT was associated with significant sparing of acute grade 2+ hematologic and grade 3+ dermatologic and gastrointestinal toxicity. Although DP-IMRT proved feasible, the high pretreatment planning revision rate emphasizes the importance of real-time radiation quality assurance for IMRT trials.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356. - PubMed
-
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin, UKCCCR Anal Cancer Trial Working Party, UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. - PubMed
-
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. - PubMed
-
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

