The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to α-synuclein toxicity in Parkinson's disease models
- PMID: 23154387
- PMCID: PMC3569987
- DOI: 10.1038/cdd.2012.142
The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to α-synuclein toxicity in Parkinson's disease models
Abstract
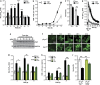
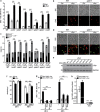
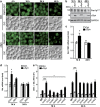
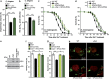
Parkinson's disease (PD) is characterized by the progressive loss of dopaminergic neurons, which arises from a yet elusive concurrence between genetic and environmental factors. The protein α-synuclein (αSyn), the principle toxic effector in PD, has been shown to interfere with neuronal Ca(2+) fluxes, arguing for an involvement of deregulated Ca(2+) homeostasis in this neuronal demise. Here, we identify the Golgi-resident Ca(2+)/Mn(2+) ATPase PMR1 (plasma membrane-related Ca(2+)-ATPase 1) as a phylogenetically conserved mediator of αSyn-driven changes in Ca(2+) homeostasis and cytotoxicity. Expression of αSyn in yeast resulted in elevated cytosolic Ca(2+) levels and increased cell death, both of which could be inhibited by deletion of PMR1. Accordingly, absence of PMR1 prevented αSyn-induced loss of dopaminergic neurons in nematodes and flies. In addition, αSyn failed to compromise locomotion and survival of flies when PMR1 was absent. In conclusion, the αSyn-driven rise of cytosolic Ca(2+) levels is pivotal for its cytotoxicity and requires PMR1.
Figures




References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Kruger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat.Genet. 1998;18:106–108. - PubMed
-
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. - PubMed
-
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann.Neurol. 2004;55:164–173. - PubMed
-
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann. Neurol. 2004;55:174–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

