Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells
- PMID: 23154388
- PMCID: PMC3569986
- DOI: 10.1038/cdd.2012.141
Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells
Abstract
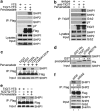
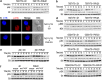
Activating and inhibitory receptors control natural killer (NK) cell activity. T-cell immunoglobulin and ITIM (immunoreceptor tyrosine-based inhibition motif) domain (TIGIT) was recently identified as a new inhibitory receptor on T and NK cells that suppressed their effector functions. TIGIT harbors the immunoreceptor tail tyrosine (ITT)-like and ITIM motifs in its cytoplasmic tail. However, how its ITT-like motif functions in TIGIT-mediated negative signaling is still unclear. Here, we show that TIGIT/PVR (poliovirus receptor) engagement disrupts granule polarization leading to loss of killing activity of NK cells. The ITT-like motif of TIGIT has a major role in its negative signaling. After TIGIT/PVR ligation, the ITT-like motif is phosphorylated at Tyr225 and binds to cytosolic adapter Grb2, which can recruit SHIP1 to prematurely terminate phosphatidylinositol 3-kinase (PI3K) and MAPK signaling, leading to downregulation of NK cell function. In support of this, Tyr225 or Asn227 mutation leads to restoration of TIGIT/PVR-mediated cytotoxicity, and SHIP1 silencing can dramatically abolish TIGIT/PVR-mediated killing inhibition.
Figures




References
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. - PubMed
-
- Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol. 2012;188:824–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

