Laminins and retinal vascular development
- PMID: 23154403
- PMCID: PMC3544790
- DOI: 10.4161/cam.22480
Laminins and retinal vascular development
Abstract
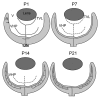
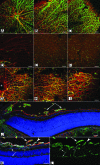
The mechanisms controlling vascular development, both normal and pathological, are not yet fully understood. Many diseases, including cancer and diabetic retinopathy, involve abnormal blood vessel formation. Therefore, increasing knowledge of these mechanisms may help develop novel therapeutic targets. The identification of novel proteins or cells involved in this process would be particularly useful. The retina is an ideal model for studying vascular development because it is easy to access, particularly in rodents where this process occurs post-natally. Recent studies have suggested potential roles for laminin chains in vascular development of the retina. This review will provide an overview of these studies, demonstrating the importance of further research into the involvement of laminins in retinal blood vessel formation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
