Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila
- PMID: 23154413
- PMCID: PMC3513996
- DOI: 10.1242/dev.087791
Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila
Abstract
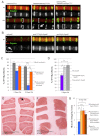
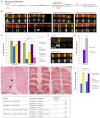
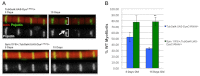
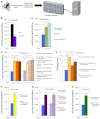
Duchenne muscular dystrophy is a lethal genetic disease characterized by the loss of muscle integrity and function over time. Using Drosophila, we show that dystrophic muscle phenotypes can be significantly suppressed by a reduction of wunen, a homolog of lipid phosphate phosphatase 3, which in higher animals can dephosphorylate a range of phospholipids. Our suppression analyses include assessing the localization of Projectin protein, a titin homolog, in sarcomeres as well as muscle morphology and functional movement assays. We hypothesize that wunen-based suppression is through the elevation of the bioactive lipid Sphingosine 1-phosphate (S1P), which promotes cell proliferation and differentiation in many tissues, including muscle. We confirm the role of S1P in suppression by genetically altering S1P levels via reduction of S1P lyase (Sply) and by upregulating the serine palmitoyl-CoA transferase catalytic subunit gene lace, the first gene in the de novo sphingolipid biosynthetic pathway and find that these manipulations also reduce muscle degeneration. Furthermore, we show that reduction of spinster (which encodes a major facilitator family transporter, homologs of which in higher animals have been shown to transport S1P) can also suppress dystrophic muscle degeneration. Finally, administration to adult flies of pharmacological agents reported to elevate S1P signaling significantly suppresses dystrophic muscle phenotypes. Our data suggest that localized intracellular S1P elevation promotes the suppression of muscle wasting in flies.
Figures


Similar articles
-
Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy.Dis Model Mech. 2014 Jan;7(1):41-54. doi: 10.1242/dmm.013631. Epub 2013 Sep 25. Dis Model Mech. 2014. PMID: 24077965 Free PMC article.
-
Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway.PLoS One. 2012;7(5):e37218. doi: 10.1371/journal.pone.0037218. Epub 2012 May 14. PLoS One. 2012. PMID: 22606352 Free PMC article.
-
Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs.J Clin Invest. 2014 Dec;124(12):5368-84. doi: 10.1172/JCI74188. Epub 2014 Oct 27. J Clin Invest. 2014. PMID: 25347472 Free PMC article.
-
S1P/S1P Receptor Signaling in Neuromuscolar Disorders.Int J Mol Sci. 2019 Dec 17;20(24):6364. doi: 10.3390/ijms20246364. Int J Mol Sci. 2019. PMID: 31861214 Free PMC article. Review.
-
Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors.Pharmacol Ther. 2000 Nov;88(2):115-31. doi: 10.1016/s0163-7258(00)00084-x. Pharmacol Ther. 2000. PMID: 11150592 Review.
Cited by
-
Adult Drosophila muscle morphometry through microCT reveals dynamics during ageing.Open Biol. 2019 Jun 28;9(6):190087. doi: 10.1098/rsob.190087. Epub 2019 Jun 26. Open Biol. 2019. PMID: 31238820 Free PMC article.
-
Silencing of drpr leads to muscle and brain degeneration in adult Drosophila.Am J Pathol. 2014 Oct;184(10):2653-61. doi: 10.1016/j.ajpath.2014.06.018. Epub 2014 Aug 8. Am J Pathol. 2014. PMID: 25111228 Free PMC article.
-
Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation.Cell Metab. 2023 May 2;35(5):855-874.e5. doi: 10.1016/j.cmet.2023.03.022. Epub 2023 Apr 20. Cell Metab. 2023. PMID: 37084732 Free PMC article.
-
Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond.Nat Rev Drug Discov. 2013 Sep;12(9):688-702. doi: 10.1038/nrd4099. Epub 2013 Aug 19. Nat Rev Drug Discov. 2013. PMID: 23954895 Free PMC article. Review.
-
Visualizing Synaptic Degeneration in Adult Drosophila in Association with Neurodegeneration.J Vis Exp. 2020 May 13;(159):10.3791/61363. doi: 10.3791/61363. J Vis Exp. 2020. PMID: 32478750 Free PMC article.
References
-
- Ayme-Southgate A., Saide J., Southgate R., Bounaix C., Cammarato A., Patel S., Wussler C. (2005). In indirect flight muscles Drosophila projectin has a short PEVK domain, and its NH2-terminus is embedded at the Z-band. J. Muscle Res. Cell Motil. 26, 467–477 - PubMed
-
- Bagdanoff J. T., Donoviel M. S., Nouraldeen A., Tarver J., Fu Q., Carlsen M., Jessop T. C., Zhang H., Hazelwood J., Nguyen H., et al. (2009). Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J. Med. Chem. 52, 3941–3953 - PubMed
-
- Bagdanoff J. T., Donoviel M. S., Nouraldeen A., Carlsen M., Jessop T. C., Tarver J., Aleem S., Dong L., Zhang H., Boteju L., et al. (2010). Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J. Med. Chem. 53, 8650–8662 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

