The angiotensin II type 1 receptor blocker olmesartan preferentially improves nocturnal hypertension and proteinuria in chronic kidney disease
- PMID: 23154587
- PMCID: PMC3594468
- DOI: 10.1038/hr.2012.184
The angiotensin II type 1 receptor blocker olmesartan preferentially improves nocturnal hypertension and proteinuria in chronic kidney disease
Abstract
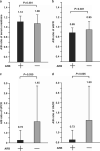
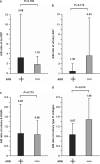
Accumulated evidence suggests that an altered ambulatory blood pressure (BP) profile, particularly elevated nighttime BP, reflects target organ injury and is a better predictor of further cardiorenal risk than the clinic BP or daytime BP in hypertensive patients complicated by chronic kidney disease (CKD). In this study, we examined the beneficial effects of olmesartan, an angiotensin II type 1 receptor blocker (ARB), on ambulatory BP profiles and renal function in hypertensive CKD patients. Forty-six patients were randomly assigned to the olmesartan add-on group (n=23) or the non-ARB group (n=23). At baseline and after the 16-week treatment period, ambulatory BP monitoring was performed and renal function parameter measurements were collected. Although the baseline clinic BP levels and the after-treatment/baseline (A/B) ratios of clinic BP levels were similar in the olmesartan add-on and non-ARB groups, the A/B ratios of ambulatory 24-h and nighttime BP levels in the olmesartan add-on group were significantly lower. Furthermore, the A/B ratios of urinary protein, albumin and type IV collagen excretion in the olmesartan add-on group were significantly lower than those in the non-ARB group (urinary protein excretion, 0.72±0.41 vs. 1.45±1.48, P=0.030; urinary albumin excretion, 0.73±0.37 vs. 1.50±1.37, P=0.005; urinary type IV collagen excretion, 0.87±0.42 vs. 1.48±0.87, P=0.014) despite comparable A/B ratios for the estimated glomerular filtration rate in the two groups. These results indicate that in hypertensive patients with CKD, olmesartan add-on therapy improves the ambulatory BP profile via a preferential reduction in nighttime BP with concomitant renal injury inhibition.
Figures
Comment in
-
How important is it to control nocturnal hypertension with angiotensin II type 1 receptor blockers?Hypertens Res. 2013 Mar;36(3):194-5. doi: 10.1038/hr.2012.188. Epub 2012 Nov 22. Hypertens Res. 2013. PMID: 23171955 No abstract available.
References
-
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. - PubMed
-
- Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–2033. - PubMed
-
- Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, Hodgkinson J, Mant J, Martin U, Williams B, Wonderling D, McManus RJ. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
