Antitumor effects of synthetic 6,7-annulated-4-substituted indole compounds in L1210 leukemic cells in vitro
- PMID: 23155229
- PMCID: PMC4175989
Antitumor effects of synthetic 6,7-annulated-4-substituted indole compounds in L1210 leukemic cells in vitro
Abstract
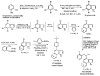
Background: Because annulated indoles have almost no representation in the PubChem or MLSMR databases, an unprecedented class of an indole-based library was constructed, using the indole aryne methodology, and screened for antitumor activity. Sixty-six novel 6,7-annulated-4-substituted indole compounds were synthesized, using a strategic combination of 6,7-indolyne cycloaddition and cross-coupling reactions under both Suzuki-Miyaura and Buchwald-Hartwig conditions, and tested for their effectiveness against murine L1210 tumor cell proliferation in vitro.
Materials and methods: Various markers of tumor cell metabolism, DNA degradation, mitotic disruption, cytokinesis and apoptosis were assayed in vitro to evaluate drug cytotoxicity.
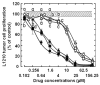
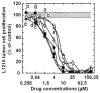
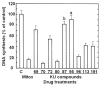
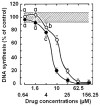
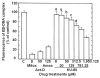
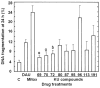
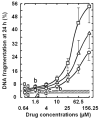
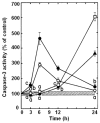
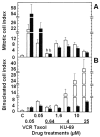
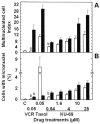
Results: Most compounds inhibited the metabolic activity of leukemic cells in a time- and concentration-dependent manner but only 9 of them were sufficiently potent to inhibit L1210 tumor cell proliferation by 50% in the low-μM range after 2 (IC(50): 4.5-20.4 μM) and 4 days (0.5-4.0 μM) in culture. However, the antiproliferative compounds that were the most effective at day 4 were not necessarily the most potent at day 2, suggesting different speeds of action. A 3-h treatment with antiproliferative annulated indole was sufficient to inhibit, in a concentration-dependent manner, the rate of DNA synthesis measured in L1210 cells over a 0.5-h period of pulse-labeling with (3)H-thymidine. Four of the antiproliferative compounds had weak DNA-binding activities but one compound reduced the fluorescence of the ethidium bromide-DNA complex by up to 53%, suggesting that some annulated indoles might directly interact with double-stranded DNA to disrupt its integrity and prevent the dye from intercalating into DNA base pairs. However, all 9 antiproliferative compounds induced DNA cleavage at 24 h in L1210 cells, containing (3)H-thymidine-prelabeled DNA, suggesting that these antitumor annulated indoles might trigger an apoptotic pathway of DNA fragmentation. Indeed the antiproliferative annulated indoles caused a time-dependent increase of caspase-3 activity with a peak at 6 h. Interestingly, the compounds with the most potent antiproliferative IC(50) values at day 2 were consistently the most effective at inhibiting DNA synthesis at 3 h and inducing DNA fragmentation at 24 h. After 24-48 h, antiproliferative concentrations of annulated indoles increased the mitotic index of L1210 cells and stimulated the formation of many bi-nucleated cells, multi-nucleated cells, apoptotic cells and micronuclei, suggesting that these antitumor compounds might enhance mitotic abnormality, induce chromosomal damage or missegregation, and block cytokinesis to induce apoptosis.
Conclusion: Although annulated indoles may have interesting bioactivity, novel derivatives with different substitutions must be synthesized to elucidate structure-activity relationships, identify more potent antitumor lead compounds, and investigate their molecular targets and mechanisms of action.
Figures
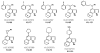
Similar articles
-
Mechanisms by which synthetic 6,7-annulated-4-substituted indole compounds with anti-proliferative activity disrupt mitosis and block cytokinesis in human HL-60 tumor cells in vitro.Anticancer Res. 2014 Apr;34(4):1643-55. Anticancer Res. 2014. PMID: 24692693 Free PMC article.
-
Bioactivity of synthetic 2-halo-3-aryl-4(3H)-quinazoliniminium halides in L1210 leukemia and SK-BR-3 mammary tumor cells in vitro.Anticancer Res. 2011 Jun;31(6):2083-93. Anticancer Res. 2011. PMID: 21737626
-
Bioactivity and molecular targets of novel substituted quinolines in murine and human tumor cell lines in vitro.Int J Oncol. 2010 Mar;36(3):673-88. doi: 10.3892/ijo_00000543. Int J Oncol. 2010. PMID: 20126988
-
The importance of indole and azaindole scaffold in the development of antitumor agents.Eur J Med Chem. 2020 Oct 1;203:112506. doi: 10.1016/j.ejmech.2020.112506. Epub 2020 Jun 28. Eur J Med Chem. 2020. PMID: 32688198 Review.
-
Antiproliferative Effect of Indole Phytoalexins.Molecules. 2016 Nov 26;21(12):1626. doi: 10.3390/molecules21121626. Molecules. 2016. PMID: 27898039 Free PMC article. Review.
Cited by
-
Biomedical importance of indoles.Molecules. 2013 Jun 6;18(6):6620-62. doi: 10.3390/molecules18066620. Molecules. 2013. PMID: 23743888 Free PMC article. Review.
-
A Practical, Multi-gram Synthesis of (±)-Herbindole A, (±)-Herbindole B, and (±)-Herbindole C from a Common Intermediate via 6,7-Indole Aryne Cycloaddition and Pd(0)-Catalyzed Cross-Coupling Reactions.Tetrahedron Lett. 2015 Jun 3;56(23):3507-3510. doi: 10.1016/j.tetlet.2015.02.064. Tetrahedron Lett. 2015. PMID: 26516291 Free PMC article.
-
Mechanisms by which synthetic 6,7-annulated-4-substituted indole compounds with anti-proliferative activity disrupt mitosis and block cytokinesis in human HL-60 tumor cells in vitro.Anticancer Res. 2014 Apr;34(4):1643-55. Anticancer Res. 2014. PMID: 24692693 Free PMC article.
References
-
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Indole-derived arynes and their Diels-Alder reactivity with furans. Org Lett. 2007;9:4135–4137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
