Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways
- PMID: 23155381
- PMCID: PMC3498304
- DOI: 10.1371/journal.pone.0048299
Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways
Abstract
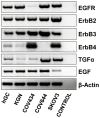
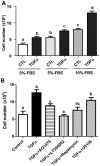
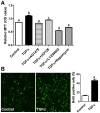
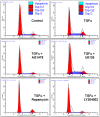
Granulosa cell tumors (GCTs) are the most common ovarian estrogen producing tumors, leading to symptoms of excessive estrogen such as endometrial hyperplasia and endometrial adenocarcinoma. These tumors have malignant potential and often recur. The etiology of GCT is unknown. TGFα is a potent mitogen for many different cells. However, its function in GCT initiation, progression and metastasis has not been determined. The present study aims to determine whether TGFα plays a role in the growth of GCT cells. KGN cells, which are derived from an invasive GCT and have many features of normal granulosa cells, were used as the cellular model. Immunohistochemistry, Western blot and RT-PCR results showed that the ErbB family of receptors is expressed in human GCT tissues and GCT cell lines. RT-PCR results also indicated that TGFα and EGF are expressed in the human granulosa cells and the GCT cell lines, suggesting that TGFα might regulate GCT cell function in an autocrine/paracrine manner. TGFα stimulated KGN cell DNA synthesis, cell proliferation, cell viability, cell cycle progression, and cell migration. TGFα rapidly activated EGFR/PI3K/Akt and mTOR pathways, as indicated by rapid phosphorylation of Akt, TSC2, Rictor, mTOR, P70S6K and S6 proteins following TGFα treatment. TGFα also rapidly activated the EGFR/MEK/ERK pathway, and P38 MAPK pathways, as indicated by the rapid phosphorylation of EGFR, MEK, ERK1/2, P38, and CREB after TGFα treatment. Whereas TGFα triggered a transient activation of Akt, it induced a sustained activation of ERK1/2 in KGN cells. Long-term treatment of KGN cells with TGFα resulted in a significant increase in cyclin D2 and a decrease in p27/Kip1, two critical regulators of granulosa cell proliferation and granulosa cell tumorigenesis. In conclusion, TGFα, via multiple signaling pathways, regulates KGN cell proliferation and migration and may play an important role in the growth and metastasis of GCTs.
Conflict of interest statement
Figures






References
-
- Colombo N, Parma G, Zanagnolo V, Insinga A (2007) Management of ovarian stromal cell tumors. J Clin Oncol 25: 2944–2951. - PubMed
-
- Fuller PJ, Chu S (2004) Signalling pathways in the molecular pathogenesis of ovarian granulosa cell tumours. Trends Endocrinol Metab 15: 122–128. - PubMed
-
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, et al. (2001) Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142: 437–445. - PubMed
-
- Schumer ST, Cannistra SA (2003) Granulosa cell tumor of the ovary. J Clin Oncol 21: 1180–1189. - PubMed
-
- Crew KD, Cohen MH, Smith DH, Tiersten AD, Feirt NM, et al. (2005) Long natural history of recurrent granulosa cell tumor of the ovary 23 years after initial diagnosis: a case report and review of the literature. Gynecol Oncol 96: 235–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

