Plac8 is required for white adipocyte differentiation in vitro and cell number control in vivo
- PMID: 23155406
- PMCID: PMC3498234
- DOI: 10.1371/journal.pone.0048767
Plac8 is required for white adipocyte differentiation in vitro and cell number control in vivo
Abstract
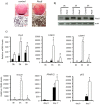
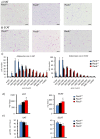
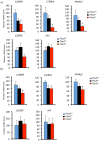
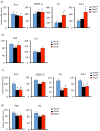
Plac8 belongs to an evolutionary conserved family of proteins, mostly abundant in plants where they control fruit weight through regulation of cell number. In mice, Plac8 is expressed both in white and brown adipose tissues and we previously showed that Plac8(-/-) mice develop late-onset obesity, with abnormal brown fat differentiation and reduced thermogenic capacity. We also showed that in brown adipocytes, Plac8 is an upstream regulator of C/EBPβ expression. Here, we first assessed the role of Plac8 in white adipogenesis in vitro. We show that Plac8 is induced early after induction of 3T3-L1 adipocytes differentiation, a process that is prevented by Plac8 knockdown; similarly, embryonic fibroblasts obtained from Plac8 knockout mice failed to form adipocytes upon stimulation of differentiation. Knockdown of Plac8 in 3T3-L1 was associated with reduced expression of C/EBPβ, Krox20, and Klf4, early regulators of the white adipogenic program, and we show that Plac8 could transactivate the C/EBPβ promoter. In vivo, we show that absence of Plac8 led to increased white fat mass with enlarged adipocytes but reduced total number of adipocytes. Finally, even though Plac8(-/-) mice showed impaired thermogenesis due to brown fat dysfunction, this was not associated with changes in glycemia or plasma free fatty acid and triglyceride levels. Collectively, these data indicate that Plac8 is an upstream regulator of C/EBPβ required for adipogenesis in vitro. However, in vivo, Plac8 is dispensable for the differentiation of white adipocytes with preserved fat storage capacity but is required for normal fat cell number regulation.
Conflict of interest statement
Figures




Similar articles
-
Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight.Cell Metab. 2011 Nov 2;14(5):658-70. doi: 10.1016/j.cmet.2011.08.008. Epub 2011 Oct 6. Cell Metab. 2011. PMID: 21982742
-
Role of C/EBPβ-LAP and C/EBPβ-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes.Differentiation. 2013 Jan;85(1-2):20-31. doi: 10.1016/j.diff.2012.11.001. Epub 2013 Jan 11. Differentiation. 2013. PMID: 23314288
-
Histone demethylase KDM5A is transactivated by the transcription factor C/EBPβ and promotes preadipocyte differentiation by inhibiting Wnt/β-catenin signaling.J Biol Chem. 2019 Jun 14;294(24):9642-9654. doi: 10.1074/jbc.RA119.008419. Epub 2019 May 6. J Biol Chem. 2019. PMID: 31061100 Free PMC article.
-
Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β.J Biol Chem. 2015 Jan 9;290(2):755-61. doi: 10.1074/jbc.R114.619957. Epub 2014 Dec 1. J Biol Chem. 2015. PMID: 25451943 Free PMC article. Review.
-
Regulatory mechanisms of the early phase of white adipocyte differentiation: an overview.Cell Mol Life Sci. 2022 Feb 20;79(3):139. doi: 10.1007/s00018-022-04169-6. Cell Mol Life Sci. 2022. PMID: 35184223 Free PMC article. Review.
Cited by
-
Phospholipid scramblase 1: a protein with multiple functions via multiple molecular interactors.Cell Commun Signal. 2022 Jun 1;20(1):78. doi: 10.1186/s12964-022-00895-3. Cell Commun Signal. 2022. PMID: 35650588 Free PMC article. Review.
-
The novel KLF4/PLAC8 signaling pathway regulates lung cancer growth.Cell Death Dis. 2018 May 22;9(6):603. doi: 10.1038/s41419-018-0580-3. Cell Death Dis. 2018. PMID: 29789534 Free PMC article.
-
Involvement of the Endocrine-Disrupting Chemical Bisphenol A (BPA) in Human Placentation.J Clin Med. 2020 Feb 3;9(2):405. doi: 10.3390/jcm9020405. J Clin Med. 2020. PMID: 32028606 Free PMC article.
-
Yeast expression of mammalian Onzin and fungal FCR1 suggests ancestral functions of PLAC8 proteins in mitochondrial metabolism and DNA repair.Sci Rep. 2019 Apr 29;9(1):6629. doi: 10.1038/s41598-019-43136-3. Sci Rep. 2019. PMID: 31036870 Free PMC article.
-
New animal models reveal that coenzyme Q2 (Coq2) and placenta-specific 8 (Plac8) are candidate genes for the onset of type 2 diabetes associated with obesity in rats.Mamm Genome. 2015 Dec;26(11-12):619-29. doi: 10.1007/s00335-015-9597-4. Epub 2015 Aug 22. Mamm Genome. 2015. PMID: 26296322
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

