Isolated Toll-like receptor transmembrane domains are capable of oligomerization
- PMID: 23155421
- PMCID: PMC3498381
- DOI: 10.1371/journal.pone.0048875
Isolated Toll-like receptor transmembrane domains are capable of oligomerization
Abstract
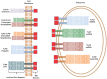
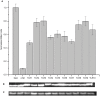
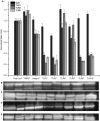
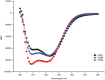
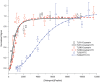
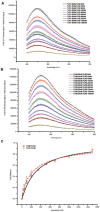
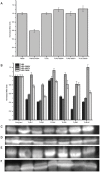
Toll-like receptors (TLRs) act as the first line of defense against bacterial and viral pathogens by initiating critical defense signals upon dimer activation. The contribution of the transmembrane domain in the dimerization and signaling process has heretofore been overlooked in favor of the extracellular and intracellular domains. As mounting evidence suggests that the transmembrane domain is a critical region in several protein families, we hypothesized that this was also the case for Toll-like receptors. Using a combined biochemical and biophysical approach, we investigated the ability of isolated Toll-like receptor transmembrane domains to interact independently of extracellular domain dimerization. Our results showed that the transmembrane domains had a preference for the native dimer partners in bacterial membranes for the entire receptor family. All TLR transmembrane domains showed strong homotypic interaction potential. The TLR2 transmembrane domain demonstrated strong heterotypic interactions in bacterial membranes with its known interaction partners, TLR1 and TLR6, as well as with a proposed interaction partner, TLR10, but not with TLR4, TLR5, or unrelated transmembrane receptors providing evidence for the specificity of TLR2 transmembrane domain interactions. Peptides for the transmembrane domains of TLR1, TLR2, and TLR6 were synthesized to further study this subfamily of receptors. These peptides validated the heterotypic interactions seen in bacterial membranes and demonstrated that the TLR2 transmembrane domain had moderately strong interactions with both TLR1 and TLR6. Combined, these results suggest a role for the transmembrane domain in Toll-like receptor oligomerization and as such, may be a novel target for further investigation of new therapeutic treatments of Toll-like receptor mediated diseases.
Conflict of interest statement
Figures
References
-
- Medzhitov R (2001) Toll-like receptors and innate immunity. Nature Reviews Immunology 1: 135–145. - PubMed
-
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology 11: 373–384. - PubMed
-
- Park BS, Song DH, Kim HM, Choi B-S, Lee H, et al. (2009) The structural basis of lipopolysacchardie regonition by the TLR4-MD-2 complex. Nature 458: 1191–1195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

