Human breast cancer cells are redirected to mammary epithelial cells upon interaction with the regenerating mammary gland microenvironment in-vivo
- PMID: 23155468
- PMCID: PMC3498340
- DOI: 10.1371/journal.pone.0049221
Human breast cancer cells are redirected to mammary epithelial cells upon interaction with the regenerating mammary gland microenvironment in-vivo
Abstract
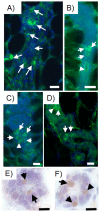
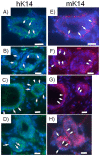
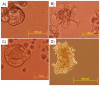
Breast cancer is the second leading cause of cancer deaths in the United States. At present, the etiology of breast cancer is unknown; however the possibility of a distinct cell of origin, i.e. a cancer stem cell, is a heavily investigated area of research. Influencing signals from the tissue niche are known to affect stem cells. Literature has shown that cancer cells lose their tumorigenic potential and display 'normal' behavior when placed into 'normal' ontogenic environments. Therefore, it may be the case that the tissue microenvironment is able to generate signals to redirect cancer cell fate. Previously, we showed that pluripotent human embryonal carcinoma cells could be redirected by the regenerating mammary gland microenvironment to contribute epithelial progeny for 'normal' gland development in-vivo. Here, we show that that human metastatic, non-metastatic, and metastasis-suppressed breast cancer cells proliferate and contribute to normal mammary gland development in-vivo without tumor formation. Immunochemistry for human-specific mitochondria, keratin 8 and 14, as well as human-specific milk proteins (alpha-lactalbumin, impregnated transplant hosts) confirmed the presence of human cell progeny. Features consistent with normal mammary gland development as seen in intact hosts (duct, lumen formation, development of secretory acini) were recapitulated in both primary and secondary outgrowths from chimeric implants. These results suggest the dominance of the tissue microenvironment over cancer cell fate. This work demonstrates that cultured human breast cancer cells (metastatic and non-metastatic) respond developmentally to signals generated by the mouse mammary gland microenvironment during gland regeneration in-vivo.
Conflict of interest statement
Figures




References
-
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. - PubMed
-
- Lorusso G, Ruegg C (2008) The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 130: 1091–1103. - PubMed
-
- Felsher DW (2003) Cancer revoked: Oncogenes as therapeutic targets. Nat Rev Cancer 3: 375–380. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

