Specificity in S-nitrosylation: a short-range mechanism for NO signaling?
- PMID: 23157283
- PMCID: PMC3785806
- DOI: 10.1089/ars.2012.5066
Specificity in S-nitrosylation: a short-range mechanism for NO signaling?
Abstract
Significance: Nitric oxide (NO) classical and less classical signaling mechanisms (through interaction with soluble guanylate cyclase and cytochrome c oxidase, respectively) operate through direct binding of NO to protein metal centers, and rely on diffusibility of the NO molecule. S-Nitrosylation, a covalent post-translational modification of protein cysteines, has emerged as a paradigm of nonclassical NO signaling.
Recent advances: Several nonenzymatic mechanisms for S-nitrosylation formation and destruction have been described. Enzymatic mechanisms for transnitrosylation and denitrosylation have been also studied as regulators of the modification of specific subsets of proteins. The advancement of modification-specific proteomic methodologies has allowed progress in the study of diverse S-nitrosoproteomes, raising clues and questions about the parameters for determining the protein specificity of the modification.
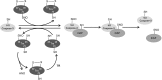
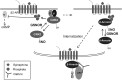
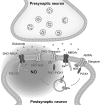
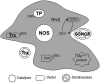
Critical issues: We propose that S-nitrosylation is mainly a short-range mechanism of NO signaling, exerted in a relatively limited range of action around the NO sources, and tightly related to the very controlled regulation of subcellular localization of nitric oxide synthases. We review the nonenzymatic and enzymatic mechanisms that support this concept, as well as physiological examples of mammalian systems that illustrate well the precise compartmentalization of S-nitrosylation.
Future directions: Individual and proteomic studies of protein S-nitrosylation-based signaling should take into account the subcellular localization in order to gain further insight into the functional role of this modification in (patho)physiological settings.
Figures


References
-
- Aarts M. Liu Y. Liu L. Besshoh S. Arundine M. Gurd JW. Wang YT. Salter MW. Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Aarts MM. Tymianski M. Molecular mechanisms underlying specificity of excitotoxic signaling in neurons. Curr Mol Med. 2004;4:137–147. - PubMed
-
- Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
-
- Ahern GP. Klyachko VA. Jackson MB. cGMP and S-nitrosylation: Two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

