The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin
- PMID: 23157335
- PMCID: PMC3965671
- DOI: 10.1146/annurev-pathol-011110-130318
The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin
Abstract
The recent discovery of a new CD4+ T cell subset, Th17, has transformed our understanding of the pathogenetic basis of an increasing number of chronic immune-mediated diseases. Particularly in tissues that interface with the microbial environment-such as the intestinal and respiratory tracts and the skin-where most of the Th17 cells in the body reside, dysregulated immunity to self (or the extended self, the diverse microbiota that normally colonize these tissues) can result in chronic inflammatory disease. In this review, we focus on recent advances in the biology of the Th17 pathway and on genome-wide association studies that implicate this immune pathway in human disease involving these tissues.
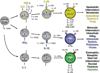
Figures





References
-
- Coffman RL. Origins of the TH1-TH2 model: a personal perspective. Nat Immunol. 2006;7:539–541. - PubMed
-
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–173. - PubMed
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology. 2007;25:821–852. - PubMed
-
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annual Review of Immunology. 2007;25:221–242. - PubMed
-
- Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Current Opinion in Immunology. 2009;21:274–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

