Defining putative glycan cancer biomarkers by MS
- PMID: 23157355
- PMCID: PMC3673031
- DOI: 10.4155/bio.12.246
Defining putative glycan cancer biomarkers by MS
Abstract
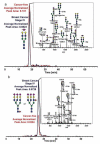
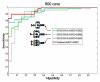
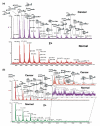
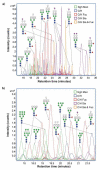
For decades, the association between aberrant glycosylation and many types of cancers has been shown. However, defining the changes of glycan structures has not been demonstrated until recently. This has been facilitated by the major advances in MS and separation science, which allows the detailed characterization of glycan changes associated with cancer. MS glycomics methods have been successfully employed to compare the glycomic profiles of different human specimens collected from disease-free individuals and patients with cancer. Additionally, comparing the glycomic profiles of glycoproteins purified from specimen collected from disease-free individuals and patients with cancer has also been performed. These types of glycan analyses employing MS or LC-MS allow the characterization of native, labeled and permethylated glycans. This review discusses the different glycomic and glycoproteomic methods employed for defining glycans as cancer biomarkers of different organs, including breast, colon, esophagus, liver, lung, ovarian, pancreas and prostate.
Figures

References
-
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. - PubMed
-
- Rudd PM, Woods RJ, Wormald MR, et al. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochem. Biophys. Acta. 1995;1248(1):1–10. - PubMed
-
- Rudd PM, Wormald MR, Stanfield RL, et al. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J. Mol. Biol. 1999;293(2):351–366. - PubMed
-
- Dwek RA. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996;96(2):683–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
