Micrometer scale guidance of mesenchymal stem cells to form structurally oriented cartilage extracellular matrix
- PMID: 23157410
- PMCID: PMC3609643
- DOI: 10.1089/ten.TEA.2012.0177
Micrometer scale guidance of mesenchymal stem cells to form structurally oriented cartilage extracellular matrix
Abstract
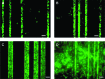
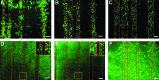
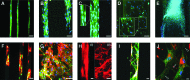
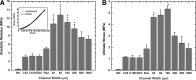
Tissue engineering is a possible method for long-term repair of cartilage lesions, but current tissue-engineered cartilage constructs have inferior mechanical properties compared to native cartilage. This problem may be due to the lack of an oriented structure in the constructs at the microscale that is present in the native tissue. In this study, we utilize contact guidance to develop constructs with microscale architecture for improved chondrogenesis and function. Stable channels of varying microscale dimensions were formed in collagen-based and polydimethylsiloxane membranes via a combination of microfabrication and soft-lithography. Human mesenchymal stem cells (MSCs) were selectively seeded in these channels. The chondrogenic potential of MSCs seeded in these channels was investigated by culturing them for 3 weeks under differentiating conditions, and then evaluating the subsequent synthesized tissue for mechanical function and by type II collagen immunohistochemistry. We demonstrate selective seeding of viable MSCs within the channels. MSC aligned and produced mature collagen fibrils along the length of the channel in smaller linear channels of widths 25-100 μm compared to larger linear channels of widths 500-1000 μm. Further, substrates with microchannels that led to cell alignment also led to superior mechanical properties compared to constructs with randomly seeded cells or selectively seeded cells in larger channels. The ultimate stress and modulus of elasticity of constructs with cells seeded in smaller channels increased by as much as fourfolds. We conclude that microscale guidance is useful to produce oriented cartilage structures with improved mechanical properties. These findings can be used to fabricate large clinically useful MSC-cartilage constructs with superior mechanical properties.
Figures




Similar articles
-
Micrometer scale guidance of mesenchymal stem cells to form structurally oriented large-scale tissue engineered cartilage.Acta Biomater. 2017 Sep 15;60:210-219. doi: 10.1016/j.actbio.2017.07.016. Epub 2017 Jul 11. Acta Biomater. 2017. PMID: 28709984 Free PMC article.
-
Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels.Osteoarthritis Cartilage. 2008 Sep;16(9):1074-82. doi: 10.1016/j.joca.2008.02.005. Epub 2008 Mar 18. Osteoarthritis Cartilage. 2008. PMID: 18353693 Free PMC article.
-
High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties.Acta Biomater. 2012 Aug;8(8):3027-34. doi: 10.1016/j.actbio.2012.04.033. Epub 2012 Apr 27. Acta Biomater. 2012. PMID: 22546516 Free PMC article.
-
Intact vitreous humor as a potential extracellular matrix hydrogel for cartilage tissue engineering applications.Acta Biomater. 2019 Feb;85:117-130. doi: 10.1016/j.actbio.2018.12.022. Epub 2018 Dec 18. Acta Biomater. 2019. PMID: 30572166
-
Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage.J Biomech. 2010 Jan 5;43(1):128-36. doi: 10.1016/j.jbiomech.2009.09.018. Epub 2009 Oct 13. J Biomech. 2010. PMID: 19828149 Free PMC article. Review.
Cited by
-
Micrometer scale guidance of mesenchymal stem cells to form structurally oriented large-scale tissue engineered cartilage.Acta Biomater. 2017 Sep 15;60:210-219. doi: 10.1016/j.actbio.2017.07.016. Epub 2017 Jul 11. Acta Biomater. 2017. PMID: 28709984 Free PMC article.
-
Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells.PLoS One. 2014 Oct 7;9(10):e109811. doi: 10.1371/journal.pone.0109811. eCollection 2014. PLoS One. 2014. PMID: 25290150 Free PMC article.
-
Material stiffness effects on neurite alignment to photopolymerized micropatterns.Biomacromolecules. 2014 Oct 13;15(10):3717-27. doi: 10.1021/bm501019s. Epub 2014 Sep 29. Biomacromolecules. 2014. PMID: 25211120 Free PMC article.
-
Spidroin striped micropattern promotes chondrogenic differentiation of human Wharton's jelly mesenchymal stem cells.Sci Rep. 2022 Mar 22;12(1):4837. doi: 10.1038/s41598-022-08982-8. Sci Rep. 2022. PMID: 35319008 Free PMC article.
-
The Effect of Biomolecular Gradients on Mesenchymal Stem Cell Chondrogenesis under Shear Stress.Micromachines (Basel). 2015 Mar;6(3):330-346. doi: 10.3390/mi6030330. Epub 2015 Mar 2. Micromachines (Basel). 2015. PMID: 34026281 Free PMC article.
References
-
- CDC. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation–United States, 2007–2009. Morbid Mortal Week Rep. 2010;59:1261. - PubMed
-
- Friedenstein A.J. Marrow stromal fibroblasts. Calcif Tissue Int. 1995;56:S17. - PubMed
-
- Caplan A.I. Elyaderani M. Mochizuki Y. Wakitani S. Goldberg V.M. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997;342:254. - PubMed
-
- Caplan A.I. Goldberg V.M. Orthopaedic tissue engineering—editorial comment. Clin Orthop Relat Res. 1999;367:S2.
-
- Gilbert J.E. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

