Functions of microRNAs in cardiovascular biology and disease
- PMID: 23157557
- PMCID: PMC5215839
- DOI: 10.1146/annurev-physiol-030212-183737
Functions of microRNAs in cardiovascular biology and disease
Abstract
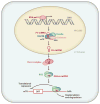
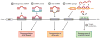
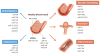
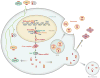
In 1993, lin-4 was discovered as a critical modulator of temporal development in Caenorhabditis elegans and, most notably, as the first in the class of small, single-stranded noncoding RNAs now defined as microRNAs (miRNAs). Another eight years elapsed before miRNA expression was detected in mammalian cells. Since then, explosive advancements in the field of miRNA biology have elucidated the basic mechanism of miRNA biogenesis, regulation, and gene-regulatory function. The discovery of this new class of small RNAs has augmented the complexity of gene-regulatory programs as well as the understanding of developmental and pathological processes in the cardiovascular system. Indeed, the contributions of miRNAs in cardiovascular development and function have been widely explored, revealing the extensive role of these small regulatory RNAs in cardiovascular physiology.
Figures



References
-
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–62. - PubMed
-
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. - PubMed
-
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–58. - PubMed
-
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

