ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR
- PMID: 23158001
- PMCID: PMC4053135
- DOI: 10.1186/bcr3358
ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR
Abstract
Introduction: Epithelial to mesenchymal transition (EMT) is associated with the basal-like breast cancer phenotypes. 60% of basal-like cancers have been shown to express wild-type estrogen receptor beta (ERbeta1). However, it is still unclear whether the ERbeta expression is related to EMT, invasion and metastasis in breast cancer. In the present study, we examined whether ERbeta1 through regulating EMT can influence invasion and metastasis in basal-like cancers.
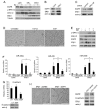
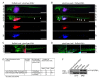
Methods: Basal-like breast cancer cells (MDA-MB-231 and Hs578T) in which ERbeta1 was either overexpressed or downregulated were analyzed for their ability to migrate and invade (wound-healing assay, matrigel-coated Transwell assay) as well as for the expression of EMT markers and components of the EGFR pathway (immunoblotting, RT-PCR). Coimmunoprecipitation and ubiquitylation assays were employed to examine whether ERbeta1 alters EGFR protein degradation and the interaction between EGFR and the ubiquitin ligase c-Cbl. The metastatic potential of the ERbeta1-expressing MDA-MB-231 cells was evaluated in vivo in a zebrafish xenotransplantation model and the correlation between ERbeta1 and E-cadherin expression was examined in 208 clinical breast cancer specimens by immunohistochemistry.
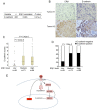
Results: Here we show that ERbeta1 inhibits EMT and invasion in basal-like breast cancer cells when they grow either in vitro or in vivo in zebrafish. The inhibition of EMT correlates with an ERbeta1-mediated upregulation of miR-200a/b/429 and the subsequent repression of ZEB1 and SIP1, which results in increased expression of E-cadherin. The positive correlation of ERbeta1 and E-cadherin expression was additionally observed in breast tumor samples. Downregulation of the basal marker EGFR through stabilization of the ubiquitin ligase c-Cbl complexes and subsequent ubiquitylation and degradation of the activated receptor is involved in the ERbeta1-mediated repression of EMT and induction of EGFR signaling abolished the ability of ERbeta1 to sustain the epithelial phenotype.
Conclusions: Taken together, the results of our study strengthen the association of ERbeta1 with the regulation of EMT and propose the receptor as a potential crucial marker in predicting metastasis in breast cancer.
Figures



Similar articles
-
IBP regulates epithelial-to-mesenchymal transition and the motility of breast cancer cells via Rac1, RhoA and Cdc42 signaling pathways.Oncogene. 2014 Jun 26;33(26):3374-82. doi: 10.1038/onc.2013.337. Epub 2013 Aug 26. Oncogene. 2014. PMID: 23975422 Free PMC article.
-
Restin suppressed epithelial-mesenchymal transition and tumor metastasis in breast cancer cells through upregulating mir-200a/b expression via association with p73.Mol Cancer. 2015 May 14;14:102. doi: 10.1186/s12943-015-0370-9. Mol Cancer. 2015. PMID: 25972084 Free PMC article.
-
The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2.J Biol Chem. 2008 May 30;283(22):14910-4. doi: 10.1074/jbc.C800074200. Epub 2008 Apr 14. J Biol Chem. 2008. PMID: 18411277 Free PMC article.
-
The Post-Translational Regulation of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in Cancer Metastasis.Int J Mol Sci. 2021 Mar 30;22(7):3591. doi: 10.3390/ijms22073591. Int J Mol Sci. 2021. PMID: 33808323 Free PMC article. Review.
-
Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer.J Transl Med. 2020 Feb 3;18(1):51. doi: 10.1186/s12967-020-02240-z. J Transl Med. 2020. PMID: 32014049 Free PMC article. Review.
Cited by
-
RNF208, an estrogen-inducible E3 ligase, targets soluble Vimentin to suppress metastasis in triple-negative breast cancers.Nat Commun. 2019 Dec 20;10(1):5805. doi: 10.1038/s41467-019-13852-5. Nat Commun. 2019. PMID: 31862882 Free PMC article.
-
Advancements in Zebrafish Models for Breast Cancer Research: Unveiling Biomarkers, Targeted Therapies, and Personalized Medicine.Med Sci Monit. 2023 Jul 2;29:e940550. doi: 10.12659/MSM.940550. Med Sci Monit. 2023. PMID: 37393427 Free PMC article.
-
Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy.J Exp Clin Cancer Res. 2019 Aug 14;38(1):354. doi: 10.1186/s13046-019-1359-9. J Exp Clin Cancer Res. 2019. PMID: 31412908 Free PMC article.
-
Somatic loss of estrogen receptor beta and p53 synergize to induce breast tumorigenesis.Breast Cancer Res. 2017 Jul 3;19(1):79. doi: 10.1186/s13058-017-0872-z. Breast Cancer Res. 2017. PMID: 28673316 Free PMC article.
-
Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies.Cancers (Basel). 2020 Aug 24;12(9):2392. doi: 10.3390/cancers12092392. Cancers (Basel). 2020. PMID: 32846967 Free PMC article. Review.
References
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;14:747–752. doi: 10.1038/35021093. - DOI - PubMed
-
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Graf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Langerod A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Borresen-Dale AL, Brenton JD, Tavare S, Caldas C. et al.The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;14:346–352. - PMC - PubMed
-
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;14:8418–8423. doi: 10.1073/pnas.0932692100. - DOI - PMC - PubMed
-
- Rakha E, Ellis I, Reis-Filho J. Are triple-negative and basal-like breast cancer synonymous? Clin Cancer Res. 2008;14:618–619. author reply 618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

