Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans
- PMID: 23159449
- PMCID: PMC3835150
- DOI: 10.1053/j.gastro.2012.11.022
Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans
Abstract
Background & aims: Roux-en-Y gastric bypass (RYGB) improves glucose homeostasis independently of changes in body weight by unknown mechanisms. Melanocortin-4 receptors (MC4R) have weight-independent effects on glucose homeostasis, via autonomic neurons, and also might contribute to weight loss after RYGB. We investigated whether MC4Rs mediate effects of RYGB, such as its weight-independent effects on glucose homeostasis, in mice and humans.
Methods: We studied C57BL/6 mice with diet-induced obesity, MC4R-deficient mice, and mice that re-express MC4R specifically in autonomic neurons after RYGB or sham surgeries. We also sequenced the MC4R locus in patients undergoing RYGB to investigate diabetes resolution in carriers of rare MC4R variants.
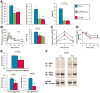
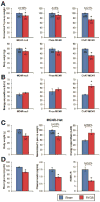
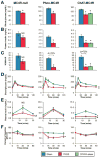
Results: MC4Rs in autonomic brainstem neurons (including the parasympathetic dorsal motor vagus) mediated improved glucose homeostasis independent of changes in body weight. In contrast, MC4Rs in cholinergic preganglionic motor neurons (sympathetic and parasympathetic) mediated RYGB-induced increased energy expenditure and weight loss. Increased energy expenditure after RYGB is the predominant mechanism of weight loss and confers resistance to weight gain from a high-fat diet, the effects of which are MC4R-dependent. MC4R-dependent effects of RYGB still occurred in mice with Mc4r haplosufficiency, and early stage diabetes resolved at a similar rate in patients with rare variants of MC4R and noncarriers. However, carriers of MC4R (I251L), a rare variant associated with increased weight loss after RYGB and increased basal activity in vitro, were more likely to have early and weight-independent resolution of diabetes than noncarriers, indicating a role for MC4Rs in the effects of RYGB.
Conclusions: MC4Rs in autonomic neurons mediate beneficial effects of RYGB, including weight-independent improved glucose homeostasis, in mice and humans.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
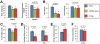


Comment in
-
Bariatric surgery in the era of personalized medicine.Gastroenterology. 2013 Mar;144(3):497-500. doi: 10.1053/j.gastro.2013.01.027. Epub 2013 Jan 21. Gastroenterology. 2013. PMID: 23347671 Free PMC article. No abstract available.
References
-
- Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. - PubMed
-
- Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15–27. - PubMed
-
- Nestoridi E, Kvas S, Kucharczyk J, et al. Resting Energy Expenditure and Energetic Cost of Feeding Are Augmented after Roux-en-Y Gastric Bypass in Obese Mice. Endocrinology. 2012;153:2234–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR002584/RR/NCRR NIH HHS/United States
- R01 DK088231/DK/NIDDK NIH HHS/United States
- P30 DK072488/DK/NIDDK NIH HHS/United States
- DK078184/DK/NIDDK NIH HHS/United States
- DK091511/DK/NIDDK NIH HHS/United States
- DK072488/DK/NIDDK NIH HHS/United States
- K08 DK091511/DK/NIDDK NIH HHS/United States
- R01 DK078184/DK/NIDDK NIH HHS/United States
- R56 DK092775/DK/NIDDK NIH HHS/United States
- R00 DA024719/DA/NIDA NIH HHS/United States
- DK092775/DK/NIDDK NIH HHS/United States
- R56 DK078184/DK/NIDDK NIH HHS/United States
- DK088231/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

