Trends in use of surgical mesh for pelvic organ prolapse
- PMID: 23159692
- PMCID: PMC3529857
- DOI: 10.1016/j.ajog.2012.11.008
Trends in use of surgical mesh for pelvic organ prolapse
Abstract
Objective: Limited data exist on the rates of pelvic organ prolapse procedures utilizing mesh. The objective of this study was to examine trends in vaginal mesh prolapse procedures (VMs), abdominal sacrocolpopexy (ASC), and minimally invasive sacrocolpopexy (MISC) from 2005 to 2010.
Study design: We utilized deidentified, adjudicated health care claims data from across the United States from 2005 to 2010. Among women 18 years old or older, we identified all mesh prolapse procedures based on current procedural terminology codes (57267 for VM, 57280 for ASC, and 57425 for MISC). VM procedures included all vaginal prolapse surgeries in which mesh was placed, whether in the anterior, apical, or posterior compartment. We estimated rates per 100,000 person-years (100,000 py) and 95% confidence intervals (CIs).
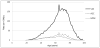
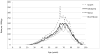
Results: During 78.5 million person-years of observation, we identified 60,152 mesh prolapse procedures, for a rate of 76.0 per 100,000 py (95% CI, 73.6-78.5). Overall, VMs comprised 74.9% of these surgeries for an overall rate of 56.9 per 100,000 py (95% CI, 55.0-58.9). Rates of ASC and MISC were considerably lower at 12.0 per 100,000 py (95% CI, 11.6-12.5) and 9.5 per 100,000 py (95% CI, 9.2-9.9), respectively. Among sacrocolpopexies, ASC was more common than MISC in 2005-2007; however, since 2007, the rate of MISC has increased, whereas the rate of ASC has decreased. Regarding trends by age, VM was considerably more common than sacrocolpopexies at all ages, and ASC was more common than MISC in women older than 50 years.
Conclusion: From 2005 to 2010, the rate of mesh prolapse procedures has increased, with vaginal mesh surgeries constituting the vast majority.
Copyright © 2013 Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure: None of the authors have a conflict of interest
Figures

References
-
- [Accessed on June 12, 2012];FDA Safety Communication: Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Issued on July 13, 2011. at http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm262435.htm. - PubMed
-
- [Accessed on June 12, 2012];FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse. Issued: October 20, 2008. at http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthno.... - PubMed
-
- American College of Obstetricians and Gynecologists and American Urogynecology Society. Committee Opinion no 513: vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118:1459–64. - PubMed
-
- FDA 510(k) Premarket Notification. AMS APOGEE VAULT SUSPENSION SYSTEM; [Accessed June 12, 2012]. Number K040537. at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=14536.
-
- FDA 510(k) Premarket Notification. AMS PERIGEE SYSTEM; [Accessed June 12, 2012]. Number K040623. at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=14611.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

