Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound
- PMID: 23159812
- PMCID: PMC3535451
- DOI: 10.1088/0031-9155/57/23/8061
Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound
Abstract
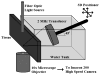
Atomization and fountain formation is a well-known phenomenon that occurs when a focused ultrasound wave in liquid encounters an air interface. High intensity focused ultrasound (HIFU) has been shown to fractionate a tissue into submicron-sized fragments in a process termed boiling histotripsy, wherein the focused ultrasound wave superheats the tissue at the focus, producing a millimetre-sized boiling or vapour bubble in several milliseconds. Yet the question of how this millimetre-sized boiling bubble creates submicron-sized tissue fragments remains. The hypothesis of this work is that the tissue can behave as a liquid such that it atomizes and forms a fountain within the vapour bubble produced in boiling histotripsy. We describe an experiment, in which a 2 MHz HIFU transducer (maximum in situ intensity of 24 000 W cm(-2)) was aligned with an air-tissue interface meant to simulate the boiling bubble. Atomization and fountain formation was observed with high-speed photography and resulted in tissue erosion. Histological examination of the atomized tissue showed whole and fragmented cells and nuclei. Air-liquid interfaces were also filmed. Our conclusion was that HIFU can fountain and atomize tissue. Although this process does not entirely mimic what was observed in liquids, it does explain many aspects of tissue fractionation in boiling histotripsy.
Figures










References
-
- Antonevich J. Ultrasonic atomization of liquids. IRE Prof Group Ultrason Eng. 1959;6:6–15.
-
- Bailey M, Khokhlova V, Sapozhnikov O, Kargl S, Crum L. Physical mechanisms of the therapeutic effect of ultrasound (A review) Acoust Phys. 2003;49:369–88.
-
- Barreras F, Amaveda H, Lozano A. Transient high-frequency ultrasonic water atomization. Exp Fluids. 2002;33:405–13.
-
- Bassett J, Bright A. Observations concerning the mechanism of atomisation in an ultrasonic fountain. J Aerosol Sci. 1976;7:47–51.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
