Estrogen receptor α is a novel target of the Von Hippel-Lindau protein and is responsible for the proliferation of VHL-deficient cells under hypoxic conditions
- PMID: 23159849
- PMCID: PMC3552928
- DOI: 10.4161/cc.22794
Estrogen receptor α is a novel target of the Von Hippel-Lindau protein and is responsible for the proliferation of VHL-deficient cells under hypoxic conditions
Abstract
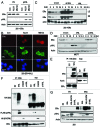
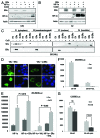
The Von Hippel-Lindau gene (VHL) is frequently deleted or mutated in human renal cell carcinoma (RCC) at the early stage. According to the well-established theory, pVHL acts as a tumor suppressor through its E3 ligase activity, which targets hypoxia-inducing factor-1α (HIF-1α). However, the elevated expression of HIF-1α did not promote cell proliferation, indicating that there would be another target, which could promote cell proliferation at the early cancer stage of RCC. In this study, we show that estrogen receptor-α (ER-α) is a novel proteasomal degradation target of the pVHL E3 ligase. Indeed, the overexpression of VHL suppresses exo- and endogenous ER-α expression, whereas si-pVHL can increase ER-α expression. The negative regulation of pVHL on ER-α expression is achieved by its E3 ligase activity. Thus, pVHL can promote the ER-α ubiquitinylation. In addition, we revealed that ER-α and HIF-1α are competitive substrates of pVHL. Thus, under normal conditions, ER-α overexpression can increase the transcription factor activity of HIF-1α. Under the hypoxic condition, where HIF-1α is not a suitable target of pVHL, ER-α is more rapidly degraded by pVHL. However, in VHL-deficient cells, the expression of ER-α and HIF-1α is retained, so that the hypoxic condition did not suppress cell proliferation obviously compared with cells that are expressing pVHL. Thus, blocking of ER-α using its inhibitor could suppress the proliferation of VHL-deficient cells as effectively as hypoxia-induced growth suppression. Considering our results, blocking of ER-α signaling in VHL-deficient cancer cells would be beneficial for cancer suppression. Indeed, we showed the anti-proliferative effect of Faslodex in VHL-deficient cells.
Figures





Similar articles
-
Elevated estrogen receptor-α in VHL-deficient condition induces microtubule organizing center amplification via disruption of BRCA1/Rad51 interaction.Neoplasia. 2014 Dec;16(12):1070-81. doi: 10.1016/j.neo.2014.09.013. Neoplasia. 2014. PMID: 25499220 Free PMC article.
-
Mutations of the von Hippel-Lindau gene confer increased susceptibility to natural killer cells of clear-cell renal cell carcinoma.Oncogene. 2011 Jun 9;30(23):2622-32. doi: 10.1038/onc.2010.638. Epub 2011 Jan 24. Oncogene. 2011. PMID: 21258414
-
Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease.Cancer Res. 2002 Jul 1;62(13):3803-11. Cancer Res. 2002. PMID: 12097293
-
Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer.Am J Nephrol. 2004 Jan-Feb;24(1):1-13. doi: 10.1159/000075346. Epub 2003 Dec 3. Am J Nephrol. 2004. PMID: 14654728 Review.
-
The von Hippel-Lindau tumor suppressor gene and kidney cancer.Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6290S-5S. doi: 10.1158/1078-0432.CCR-sup-040025. Clin Cancer Res. 2004. PMID: 15448019 Review.
Cited by
-
Cardiovascular Risk After SARS-CoV-2 Infection Is Mediated by IL18/IL18R1/HIF-1 Signaling Pathway Axis.Front Immunol. 2022 Jan 5;12:780804. doi: 10.3389/fimmu.2021.780804. eCollection 2021. Front Immunol. 2022. PMID: 35069552 Free PMC article.
-
TET is targeted for proteasomal degradation by the PHD-pVHL pathway to reduce DNA hydroxymethylation.J Biol Chem. 2020 Nov 27;295(48):16299-16313. doi: 10.1074/jbc.RA120.014538. Epub 2020 Sep 22. J Biol Chem. 2020. PMID: 32963106 Free PMC article.
-
Elevated estrogen receptor-α in VHL-deficient condition induces microtubule organizing center amplification via disruption of BRCA1/Rad51 interaction.Neoplasia. 2014 Dec;16(12):1070-81. doi: 10.1016/j.neo.2014.09.013. Neoplasia. 2014. PMID: 25499220 Free PMC article.
-
The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis.Genes (Basel). 2022 Feb 17;13(2):362. doi: 10.3390/genes13020362. Genes (Basel). 2022. PMID: 35205407 Free PMC article. Review.
-
WSB1 promotes tumor metastasis by inducing pVHL degradation.Genes Dev. 2015 Nov 1;29(21):2244-57. doi: 10.1101/gad.268128.115. Genes Dev. 2015. PMID: 26545811 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
