Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion
- PMID: 23160003
- PMCID: PMC3556477
- DOI: 10.1038/nchembio.1130
Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion
Abstract
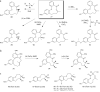
Nature has exploited medium-sized 8- to 11-membered rings in a variety of natural products to address diverse and challenging biological targets. However, owing to the limitations of conventional cyclization-based approaches to medium-ring synthesis, these structures remain severely underrepresented in current probe and drug discovery efforts. To address this problem, we have established an alternative, biomimetic ring expansion approach to the diversity-oriented synthesis of medium-ring libraries. Oxidative dearomatization of bicyclic phenols affords polycyclic cyclohexadienones that undergo efficient ring expansion to form benzannulated medium-ring scaffolds found in natural products. The ring expansion reaction can be induced using three complementary reagents that avoid competing dienone-phenol rearrangements and is driven by rearomatization of a phenol ring adjacent to the scissile bond. Cheminformatic analysis of the resulting first-generation library confirms that these molecules occupy chemical space overlapping with medium-ring natural products and distinct from that of synthetic drugs and drug-like libraries.
Figures


References
-
- Macias FA, Varela RM, Torres A, Molinillo JMG, Fronczek FR. Allelopathic studies on cultivar species. 2. Novel sesquiterpene from bioactive fractions of cultivar sunflowers. Tetrahedron Lett. 1993;34:1999–2002.
-
- Shimokawa T, Kinjo J, Yamahara J, Yamasaki M, Nohara T. Two novel aromatic compounds from Caesalpinia sappan. Chem. Pharm. Bull. 1985;33:3545–3547.
-
- Singh SB, et al. Aspercyclide A–C, three novel fungal metabolites from Aspergillus sp. as inhibitors of high-affinity IgE receptor. Tetrahedron Lett. 2004;45:7605–7608.
-
- Shirataki Y, Tagaya Y, Yokoe I, Komatsu M. Studies on the constituents of Sophora species. Part 21. Sophoraside A, a new aromatic glycoside from the roots of Sophora japonica. Chem. Pharm. Bull. 1987;35:1637–1640.
-
- Fu X, et al. Flavanone and chalcone derivatives from Cryptocarya kurzii. J. Nat. Prod. 1993;56:1153–1163. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/152163987
- PubChem-Substance/152163988
- PubChem-Substance/152163989
- PubChem-Substance/152163990
- PubChem-Substance/152163991
- PubChem-Substance/152163992
- PubChem-Substance/152163993
- PubChem-Substance/152163994
- PubChem-Substance/152163995
- PubChem-Substance/152163996
- PubChem-Substance/152163997
- PubChem-Substance/152163998
- PubChem-Substance/152163999
- PubChem-Substance/152164000
- PubChem-Substance/152164001
- PubChem-Substance/152164002
- PubChem-Substance/152164003
- PubChem-Substance/152164004
- PubChem-Substance/152164005
- PubChem-Substance/152164006
- PubChem-Substance/152164007
- PubChem-Substance/152164008
- PubChem-Substance/152164009
- PubChem-Substance/152164010
- PubChem-Substance/152164011
- PubChem-Substance/152164012
- PubChem-Substance/152164013
- PubChem-Substance/152164014
- PubChem-Substance/152164015
- PubChem-Substance/152164016
- PubChem-Substance/152164017
- PubChem-Substance/152164018
- PubChem-Substance/152164019
- PubChem-Substance/152164020
- PubChem-Substance/152164021
- PubChem-Substance/152164022
- PubChem-Substance/152164023
- PubChem-Substance/152164024
- PubChem-Substance/152164025
- PubChem-Substance/152164026
- PubChem-Substance/152164027
- PubChem-Substance/152164028
- PubChem-Substance/152164029
- PubChem-Substance/152164030
- PubChem-Substance/152164031
- PubChem-Substance/152164032
- PubChem-Substance/152164033
- PubChem-Substance/152164034
- PubChem-Substance/152164035
- PubChem-Substance/152164036
- PubChem-Substance/152164037
- PubChem-Substance/152164038
- PubChem-Substance/152164039
- PubChem-Substance/152164040
- PubChem-Substance/152164041
- PubChem-Substance/152164042
- PubChem-Substance/152164043
- PubChem-Substance/152164044
- PubChem-Substance/152164045
- PubChem-Substance/152164046
- PubChem-Substance/152164047
- PubChem-Substance/152164048
- PubChem-Substance/152164049
- PubChem-Substance/152164050
- PubChem-Substance/152164051
- PubChem-Substance/152164052
- PubChem-Substance/152164053
- PubChem-Substance/152164054
- PubChem-Substance/152164055
- PubChem-Substance/152164056
- PubChem-Substance/152164057
- PubChem-Substance/152164058
- PubChem-Substance/152164059
- PubChem-Substance/152164060
- PubChem-Substance/152164061
- PubChem-Substance/152164062
- PubChem-Substance/152164063
- PubChem-Substance/152164064
- PubChem-Substance/152164065
- PubChem-Substance/152164066
- PubChem-Substance/152164067
- PubChem-Substance/152164068
- PubChem-Substance/152164069
- PubChem-Substance/152164070
- PubChem-Substance/152164071
- PubChem-Substance/152164072
- PubChem-Substance/152164073
- PubChem-Substance/152164074
- PubChem-Substance/152164075
- PubChem-Substance/152164076
- PubChem-Substance/152164077
- PubChem-Substance/152164078
- PubChem-Substance/152164079
- PubChem-Substance/152164080
- PubChem-Substance/152164081
- PubChem-Substance/152164108
- PubChem-Substance/152164109
- PubChem-Substance/152164110
- PubChem-Substance/152164111
- PubChem-Substance/152164112
- PubChem-Substance/152164113
- PubChem-Substance/152164114
- PubChem-Substance/152164115
- PubChem-Substance/152164116
- PubChem-Substance/152164117
- PubChem-Substance/152164118
- PubChem-Substance/152164119
- PubChem-Substance/152164120
- PubChem-Substance/152164121
- PubChem-Substance/152164122
- PubChem-Substance/152164123
- PubChem-Substance/152164124
- PubChem-Substance/152164125
- PubChem-Substance/152164126
- PubChem-Substance/152164127
- PubChem-Substance/152164128
- PubChem-Substance/152164129
- PubChem-Substance/152164130
- PubChem-Substance/152164131
- PubChem-Substance/152164132
- PubChem-Substance/152164133
- PubChem-Substance/152164134
- PubChem-Substance/152164135
- PubChem-Substance/152164136
- PubChem-Substance/152164137
- PubChem-Substance/152164138
- PubChem-Substance/152164139
- PubChem-Substance/152164140
- PubChem-Substance/152164141
- PubChem-Substance/152164142
- PubChem-Substance/152164143
- PubChem-Substance/152164144
- PubChem-Substance/152164145
- PubChem-Substance/152164146
- PubChem-Substance/152164147
- PubChem-Substance/152164148
- PubChem-Substance/152164149
- PubChem-Substance/152164150
- PubChem-Substance/152164151
- PubChem-Substance/152164152
- PubChem-Substance/152164153
- PubChem-Substance/152164154
Grants and funding
LinkOut - more resources
Full Text Sources

