Use of flow cytometry for rapid, quantitative detection of poliovirus-infected cells via TAT peptide-delivered molecular beacons
- PMID: 23160127
- PMCID: PMC3553745
- DOI: 10.1128/AEM.02429-12
Use of flow cytometry for rapid, quantitative detection of poliovirus-infected cells via TAT peptide-delivered molecular beacons
Abstract
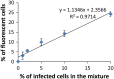
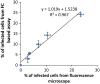
Rapid and efficient detection of viral infection is crucial for the prevention of disease spread during an outbreak and for timely clinical management. In this paper, the utility of Tat peptide-modified molecular beacons (MBs) as a rapid diagnostic tool for the detection of virus-infected cells was demonstrated. The rapid intracellular delivery mediated by the Tat peptide enabled the detection of infected cells within 30 s, reaching saturation in signal in 30 min. This rapid detection scheme was coupled with flow cytometry (FC), resulting in an automated, high-throughput method for the identification of virus-infected cells. Because of the 2-order-of-magnitude difference in fluorescence intensity between infected and uninfected cells, as few as 1% infected cells could be detected. Because of its speed and sensitivity, this approach may be adapted for the practical diagnosis of multiple viral infections.
Figures
Similar articles
-
Visualizing the dynamics of viral replication in living cells via Tat peptide delivery of nuclease-resistant molecular beacons.Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17522-5. doi: 10.1073/pnas.0807066105. Epub 2008 Nov 6. Proc Natl Acad Sci U S A. 2008. PMID: 18988730 Free PMC article.
-
Detection of infective poliovirus by a simple, rapid, and sensitive flow cytometry method based on fluorescence resonance energy transfer technology.Appl Environ Microbiol. 2010 Jan;76(2):584-8. doi: 10.1128/AEM.01851-09. Epub 2009 Nov 20. Appl Environ Microbiol. 2010. PMID: 19933336 Free PMC article.
-
Development of a particle agglutination method with soluble virus receptor for identification of poliovirus.J Clin Microbiol. 2010 Aug;48(8):2698-702. doi: 10.1128/JCM.00207-10. Epub 2010 Jun 2. J Clin Microbiol. 2010. PMID: 20519462 Free PMC article.
-
Intracellular cargo delivery using tat peptide and derivatives.Med Res Rev. 2004 Jan;24(1):1-12. doi: 10.1002/med.10056. Med Res Rev. 2004. PMID: 14595670 Review.
-
Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer.Adv Drug Deliv Rev. 2005 Feb 28;57(4):579-96. doi: 10.1016/j.addr.2004.10.005. Epub 2004 Dec 19. Adv Drug Deliv Rev. 2005. PMID: 15722165 Review.
Cited by
-
Fluorescent Protein Approaches in Alpha Herpesvirus Research.Viruses. 2015 Nov 19;7(11):5933-61. doi: 10.3390/v7112915. Viruses. 2015. PMID: 26610544 Free PMC article. Review.
References
-
- Charles PGP, Grayson ML. 2007. Point-of-care tests for lower respiratory tract infections. Med. J. Aust. 187:36–39 - PubMed
-
- Rabenau HF, Kessler HH, Kortenbusch M, Steinhorst A, Raggamb RB, Berger A. 2007. Verification and validation of diagnostic laboratory tests in clinical virology. J. Clin. Virol. 40:93–98 - PubMed
-
- Marras SAE, Kramer FR, Tyagi S. 1999. Multiplex detection of single-nucleotide variations using molecular beacons. Genet. Anal. 14:151–156 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

