Profiling in situ microbial community structure with an amplification microarray
- PMID: 23160129
- PMCID: PMC3568579
- DOI: 10.1128/AEM.02664-12
Profiling in situ microbial community structure with an amplification microarray
Abstract
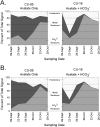
The objectives of this study were to unify amplification, labeling, and microarray hybridization chemistries within a single, closed microfluidic chamber (an amplification microarray) and verify technology performance on a series of groundwater samples from an in situ field experiment designed to compare U(VI) mobility under conditions of various alkalinities (as HCO(3)(-)) during stimulated microbial activity accompanying acetate amendment. Analytical limits of detection were between 2 and 200 cell equivalents of purified DNA. Amplification microarray signatures were well correlated with 16S rRNA-targeted quantitative PCR results and hybridization microarray signatures. The succession of the microbial community was evident with and consistent between the two microarray platforms. Amplification microarray analysis of acetate-treated groundwater showed elevated levels of iron-reducing bacteria (Flexibacter, Geobacter, Rhodoferax, and Shewanella) relative to the average background profile, as expected. Identical molecular signatures were evident in the transect treated with acetate plus NaHCO(3), but at much lower signal intensities and with a much more rapid decline (to nondetection). Azoarcus, Thaurea, and Methylobacterium were responsive in the acetate-only transect but not in the presence of bicarbonate. Observed differences in microbial community composition or response to bicarbonate amendment likely had an effect on measured rates of U reduction, with higher rates probable in the part of the field experiment that was amended with bicarbonate. The simplification in microarray-based work flow is a significant technological advance toward entirely closed-amplicon microarray-based tests and is generally extensible to any number of environmental monitoring applications.
Figures

References
-
- Handley KM, Wrighton KC, Piceno YM, Andersen GL, DeSantis TZ, Williams KH, Wilkins MJ, N′Guessan AL, Peacock A, Bargar J, Long PE, Banfield JF. 2012. High-density PhyloChip profiling of stimulated aquifer microbial communities reveals a complex response to acetate amendment. FEMS Microbiol. Ecol. 81:188–204 - PubMed
-
- Wilkins MJ, VerBerkmoes NC, Williams KH, Callister SJ, Mouser PJ, Elifantz H, N′guessan AL, Thomas BC, Nicora CD, Shah MB, Abraham P, Lipton MS, Lovley DR, Hettich RL, Long PE, Banfield JF. 2009. Proteogenomic monitoring of Geobacter physiology during stimulated uranium bioremediation. Appl. Environ. Microbiol. 75:6591–6599 - PMC - PubMed
-
- Wrighton KC, Thomas BC, Sharon I, Miller CS, Castelle CJ, VerBerkmoes NC, Wilkins MJ, Hettich RL, Lipton MS, Williams KH, Long PE, Banfield JF. 2012. Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337:1661–1665 - PubMed
-
- Waldron PJ, Wu L, Nostrand JDV, Schadt CW, He Z, Watson DB, Jardine PM, Palumbo AV, Hazen TC, Zhou J. 2009. Functional gene array-based analysis of microbial community structure in groundwaters with a gradient of contaminant levels. Environ. Sci. Technol. 43:3529–3534 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

