Canonical Wnt signaling in megakaryocytes regulates proplatelet formation
- PMID: 23160460
- PMCID: PMC3538329
- DOI: 10.1182/blood-2012-03-416875
Canonical Wnt signaling in megakaryocytes regulates proplatelet formation
Abstract
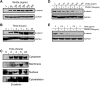
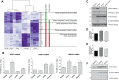
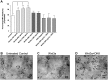
Wnt signaling is involved in numerous aspects of vertebrate development and homeostasis, including the formation and function of blood cells. Here, we show that canonical and noncanonical Wnt signaling pathways are present and functional in megakaryocytes (MKs), with several Wnt effectors displaying MK-restricted expression. Using the CHRF288-11 cell line as a model for human MKs, the canonical Wnt3a signal was found to induce a time and dose-dependent increase in β-catenin expression. β-catenin accumulation was inhibited by the canonical antagonist dickkopf-1 (DKK1) and by the noncanonical agonist Wnt5a. Whole genome expression analysis demonstrated that Wnt3a and Wnt5a regulated distinct patterns of gene expression in MKs, and revealed a further interplay between canonical and noncanonical Wnt pathways. Fetal liver cells derived from low-density-lipoprotein receptor-related protein 6-deficient mice (LRP6(-/-)), generated dramatically reduced numbers of MKs in culture of lower ploidy (2N and 4N) than wild-type controls, implicating LRP6-dependent Wnt signaling in MK proliferation and maturation. Finally, in wild-type mature murine fetal liver-derived MKs, Wnt3a potently induced proplatelet formation, an effect that could be completely abrogated by DKK1. These data identify novel extrinsic regulators of proplatelet formation, and reveal a profound role for Wnt signaling in platelet production.
Figures


References
-
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. - PubMed
-
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. - PubMed
-
- Kokolus K, Nemeth MJ. Non-canonical Wnt signaling pathways in hematopoiesis. Immunol Res. 2010;46(1-3):155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

