MicroRNA-494 reduces ATF3 expression and promotes AKI
- PMID: 23160513
- PMCID: PMC3507368
- DOI: 10.1681/ASN.2012050438
MicroRNA-494 reduces ATF3 expression and promotes AKI
Abstract
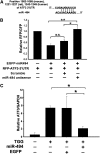
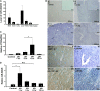
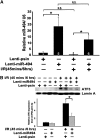
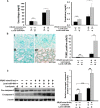
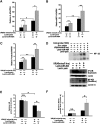
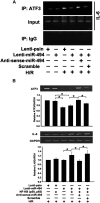
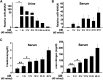
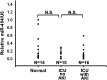
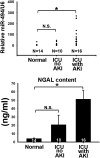
MicroRNA-494 mediates apoptosis and necrosis in several types of cells, but its renal target and potential role in AKI are unknown. Here, we found that microRNA-494 binds to the 3'UTR of activating transcription factor 3 (ATF3) and decreases its transcription. In mice, overexpression of microRNA-494 significantly attenuated the level of ATF3 and induced inflammatory mediators, such as IL-6, monocyte chemotactic protein-1, and P-selectin, after renal ischemia/reperfusion, exacerbating apoptosis and further decreasing renal function. Activation of NF-κB mediated this proinflammatory response. In this ischemia/reperfusion model, urinary levels of microRNA-494 increased significantly before the rise in serum creatinine. In humans, urinary microRNA-494 levels were 60-fold higher in patients with AKI than normal controls. In conclusion, upregulation of microRNA-494 contributes to inflammatory or adhesion molecule-induced kidney injury after ischemia/reperfusion by inhibiting expression of ATF3. Furthermore, microRNA-494 may be a specific and noninvasive biomarker for AKI.
Figures
Similar articles
-
Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion.J Cell Physiol. 2014 Sep;229(9):1202-11. doi: 10.1002/jcp.24554. J Cell Physiol. 2014. PMID: 24420912
-
Urinary interleukin-18 and urinary neutrophil gelatinase-associated lipocalin predict acute kidney injury following pulmonary valve replacement prior to serum creatinine.Congenit Heart Dis. 2012 Sep-Oct;7(5):441-7. doi: 10.1111/j.1747-0803.2012.00662.x. Epub 2012 Apr 27. Congenit Heart Dis. 2012. PMID: 22537138
-
Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study.Am J Kidney Dis. 2009 Apr;53(4):584-95. doi: 10.1053/j.ajkd.2008.10.037. Epub 2008 Dec 13. Am J Kidney Dis. 2009. PMID: 19070948
-
Biomarkers for the early detection of acute kidney injury.Pediatr Nephrol. 2008 Dec;23(12):2151-7. doi: 10.1007/s00467-007-0470-x. Epub 2007 Mar 30. Pediatr Nephrol. 2008. PMID: 17394022 Free PMC article. Review.
-
Recovery from acute kidney injury: the role of biomarkers.Nephron Clin Pract. 2014;127(1-4):101-5. doi: 10.1159/000363678. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343830 Review.
Cited by
-
Long noncoding RNA Linc01612 represses hepatocellular carcinoma progression by regulating miR-494/ATF3/p53 axis and promoting ubiquitination of YBX1.Int J Biol Sci. 2022 Apr 11;18(7):2932-2948. doi: 10.7150/ijbs.69514. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541917 Free PMC article.
-
Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications.Nat Rev Nephrol. 2019 Apr;15(4):220-239. doi: 10.1038/s41581-018-0103-6. Nat Rev Nephrol. 2019. PMID: 30651611 Free PMC article. Review.
-
Acute Kidney Injury in Kidney Transplant Patients in Intensive Care Unit: From Pathogenesis to Clinical Management.Biomedicines. 2023 May 18;11(5):1474. doi: 10.3390/biomedicines11051474. Biomedicines. 2023. PMID: 37239144 Free PMC article. Review.
-
MicroRNAs in injury and repair.Arch Toxicol. 2017 Aug;91(8):2781-2797. doi: 10.1007/s00204-017-1974-1. Epub 2017 May 13. Arch Toxicol. 2017. PMID: 28501916 Free PMC article. Review.
-
MicroRNAs as Biomarkers and Therapeutic Targets in Inflammation- and Ischemia-Reperfusion-Related Acute Renal Injury.Int J Mol Sci. 2020 Sep 14;21(18):6738. doi: 10.3390/ijms21186738. Int J Mol Sci. 2020. PMID: 32937906 Free PMC article. Review.
References
-
- Padanilam BJ: Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 - PubMed
-
- Schiffl H: Daily haemodialysis for acute renal failure. Curr Opin Nephrol Hypertens 11: 589–592, 2002 - PubMed
-
- Lovat F, Valeri N, Croce CM: MicroRNAs in the pathogenesis of cancer. Semin Oncol 38: 724–733, 2011 - PubMed
-
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M: Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

