Functional characterization of three mouse formyl peptide receptors
- PMID: 23160941
- PMCID: PMC4170117
- DOI: 10.1124/mol.112.081315
Functional characterization of three mouse formyl peptide receptors
Abstract
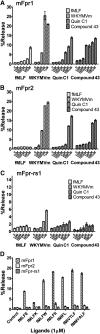
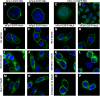
The evolutionary relationship and functional correlation between human formyl peptide receptors (FPRs) and their mouse counterparts remain incompletely understood. We examined three members of the mouse formyl peptide receptor subfamily (mFprs) and found that they differ in agonist preference and cellular distributions. When stably expressed in transfected rat basophilic leukemia (RBL-2H3) cells, mFpr1 was readily activated by N-formylated peptides derived from Listeria monocytogenes (fMIVTLF), Staphylococcus aureus (fMIFL), and mitochondria (fMMYALF). In contrast, the Escherichia coli-derived fMLF was 1000-fold less potent. The aforementioned peptides were much less efficacious at mFpr2, which responded better to the synthetic hexapeptide WKYMVm, the synthetic agonists Quin-C1 (a substituted quinazolinone), and compound 43 (a nitrosylated pyrazolone derivative). Saturation binding assays showed that mFpr1 and mFpr2 were expressed at similar levels on the cell surface, although their affinity for N-formyl-Met-Leu-Phe-Ile-Ile-Lys-fluorescein isothiocyanate varied by more than 1000-fold [dissociation constant (K(d)) values of 2.8 nM for mFpr1 and 4.8 μM for mFpr2]). Contrary to these receptors, mFpr-rs1 responded poorly to all the previously mentioned peptides that were tested. Fluorescent microscopy revealed an intracellular distribution pattern of mFpr-rs1. On the basis of these results, we conclude that mFpr1 is an ortholog of human FPR1 with certain pharmacologic properties of human FPR2/ALX, whereas mFpr2 has much lower affinity for formyl peptides. The intracellular distribution of mFpr-rs1 suggests an evolutionary correlation with human FPR3.
Figures




References
-
- Bürli RW, Xu H, Zou X, et al. (2006) Potent hFPRL1 (ALXR) agonists as potential anti-inflammatory agents. Bioorg Med Chem Lett 16:3713–3718 - PubMed
-
- Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. (2006) The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev 58:463–487 - PubMed
-
- Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D’Acquisto F, Buckingham JC, Perretti M, Flower RJ. (2010) Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol 184:2611–2619 - PMC - PubMed
-
- Forsman H, Onnheim K, Andreasson E, Dahlgren C. (2011) What formyl peptide receptors, if any, are triggered by compound 43 and lipoxin A4? Scand J Immunol 74:227–234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

