Interaction of D₃ preferring agonist (-)-N⁶-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-N⁶-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (D-264) with cloned human D₂L, D₂S, and D₃ receptors: potent stimulation of mitogen-activated protein kinases and G protein-coupled inward rectifier potassium channels
- PMID: 23160988
- PMCID: PMC3560311
- DOI: 10.1007/s00210-012-0811-6
Interaction of D₃ preferring agonist (-)-N⁶-(2-(4-(biphenyl-4-yl)piperazin-1-yl)ethyl)-N⁶-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (D-264) with cloned human D₂L, D₂S, and D₃ receptors: potent stimulation of mitogen-activated protein kinases and G protein-coupled inward rectifier potassium channels
Abstract
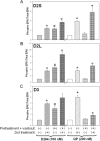
This study aims to determine the effect of the novel D(3) dopamine receptor agonist, D-264, on activation of D(3) and D(2) dopamine receptor signal transduction pathways and cell proliferation. AtT-20 neuroendocrine cells stably expressing human D(2S), D(2L), and D(3) dopamine receptors were treated with D-264 and the coupling of the receptors to mitogen-activated protein kinase (MAPK) and G protein-coupled inward rectifier potassium (GIRK) channels was determined using Western blotting and whole-cell voltage clamp recording, respectively. D-264 potently activated MAPK signaling pathway coupled to D(2S), D(2L), and D(3) dopamine receptors. The activation of MAPK was more pronounced than the reference agonist quinpirole and was longer lasting. D-264 also activated GIRK channels coupled to D(2S), D(2L), and D(3) receptors. In addition, D-264 dose-dependently induced cell proliferation in AtT-D(2L) and AtT-D(3) cells. These results indicate that D-264 robustly activates GIRK channels and MAPK coupled to D(2) and D(3) dopamine receptors in AtT-20 cells. D-264 is also a potent inducer of cell proliferation.
Figures




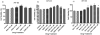
Similar articles
-
Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines.Mol Cell Neurosci. 1998 Dec;12(6):390-402. doi: 10.1006/mcne.1998.0722. Mol Cell Neurosci. 1998. PMID: 9888991
-
Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways.Neuropsychopharmacology. 2007 Jan;32(1):67-77. doi: 10.1038/sj.npp.1301071. Epub 2006 Mar 22. Neuropsychopharmacology. 2007. PMID: 16554739
-
Development of (S)-N6-(2-(4-(isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine and its analogue as a D3 receptor preferring agonist: potent in vivo activity in Parkinson's disease animal models.J Med Chem. 2010 Feb 11;53(3):1023-37. doi: 10.1021/jm901184n. J Med Chem. 2010. PMID: 20038106 Free PMC article.
-
Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states.Mol Pharmacol. 2004 Jul;66(1):97-105. doi: 10.1124/mol.66.1.97. Mol Pharmacol. 2004. PMID: 15213300
-
G Protein-Gated Potassium Channels: A Link to Drug Addiction.Trends Pharmacol Sci. 2017 Apr;38(4):378-392. doi: 10.1016/j.tips.2017.01.007. Epub 2017 Feb 7. Trends Pharmacol Sci. 2017. PMID: 28188005 Free PMC article. Review.
Cited by
-
Advances and challenges in the search for D2 and D3 dopamine receptor-selective compounds.Cell Signal. 2018 Jan;41:75-81. doi: 10.1016/j.cellsig.2017.07.003. Epub 2017 Jul 14. Cell Signal. 2018. PMID: 28716664 Free PMC article. Review.
-
The multifunctional dopamine D2/D3 receptor agonists also possess inhibitory activity against the full-length tau441 protein aggregation.Bioorg Med Chem. 2020 Sep 15;28(18):115667. doi: 10.1016/j.bmc.2020.115667. Epub 2020 Jul 28. Bioorg Med Chem. 2020. PMID: 32828429 Free PMC article.
References
-
- Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxyphenylalanine (= DOPA) on akinesia in parkinsonism. Wien Klin Wochenschr. 2001;113:851–854. 1961. - PubMed
-
- Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphth alen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008a;51:3005–3019. - PubMed
-
- Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. Further structure activity relationships study of hybrid 7-{[2-(4-Phenyl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol analogues : Identification of a high affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008b;51:101–117. - PubMed
-
- Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. - PubMed
-
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969;280:337–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

