Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose
- PMID: 23161026
- PMCID: PMC3554008
- DOI: 10.1128/JB.01789-12
Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose
Abstract
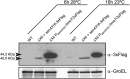
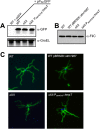
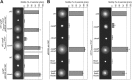
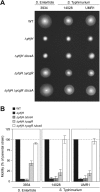
Cyclic di-GMP (c-di-GMP) is a secondary messenger that controls a variety of cellular processes, including the switch between a biofilm and a planktonic bacterial lifestyle. This nucleotide binds to cellular effectors in order to exert its regulatory functions. In Salmonella, two proteins, BcsA and YcgR, both of them containing a c-di-GMP binding PilZ domain, are the only known c-di-GMP receptors. BcsA, upon c-di-GMP binding, synthesizes cellulose, the main exopolysaccharide of the biofilm matrix. YcgR is dedicated to c-di-GMP-dependent inhibition of motility through its interaction with flagellar motor proteins. However, previous evidences indicate that in the absence of YcgR, there is still an additional element that mediates motility impairment under high c-di-GMP levels. Here we have uncovered that cellulose per se is the factor that further promotes inhibition of bacterial motility once high c-di-GMP contents drive the activation of a sessile lifestyle. Inactivation of different genes of the bcsABZC operon, mutation of the conserved residues in the RxxxR motif of the BcsA PilZ domain, or degradation of the cellulose produced by BcsA rescued the motility defect of ΔycgR strains in which high c-di-GMP levels were reached through the overexpression of diguanylate cyclases. High c-di-GMP levels provoked cellulose accumulation around cells that impeded flagellar rotation, probably by means of steric hindrance, without affecting flagellum gene expression, exportation, or assembly. Our results highlight the relevance of cellulose in Salmonella lifestyle switching as an architectural element that is both essential for biofilm development and required, in collaboration with YcgR, for complete motility inhibition.
Figures


Comment in
-
Bacterial wheel locks: extracellular polysaccharide inhibits flagellar rotation.J Bacteriol. 2013 Feb;195(3):409-10. doi: 10.1128/JB.02147-12. Epub 2012 Nov 30. J Bacteriol. 2013. PMID: 23204465 Free PMC article. No abstract available.
Similar articles
-
The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria.J Biol Chem. 2006 Oct 13;281(41):30310-4. doi: 10.1074/jbc.C600179200. Epub 2006 Aug 18. J Biol Chem. 2006. PMID: 16920715
-
The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP.Mol Microbiol. 2012 Dec;86(6):1424-40. doi: 10.1111/mmi.12066. Epub 2012 Nov 19. Mol Microbiol. 2012. PMID: 23163901 Free PMC article.
-
Under Elevated c-di-GMP in Escherichia coli, YcgR Alters Flagellar Motor Bias and Speed Sequentially, with Additional Negative Control of the Flagellar Regulon via the Adaptor Protein RssB.J Bacteriol. 2019 Dec 6;202(1):e00578-19. doi: 10.1128/JB.00578-19. Print 2019 Dec 6. J Bacteriol. 2019. PMID: 31611290 Free PMC article.
-
Emerging paradigms for PilZ domain-mediated C-di-GMP signaling.Biochem Soc Trans. 2019 Feb 28;47(1):381-388. doi: 10.1042/BST20180543. Epub 2019 Feb 1. Biochem Soc Trans. 2019. PMID: 30710060 Review.
-
Targeting cyclic di-GMP signalling: a strategy to control biofilm formation?Curr Pharm Des. 2015;21(1):12-24. doi: 10.2174/1381612820666140905124701. Curr Pharm Des. 2015. PMID: 25189859 Review.
Cited by
-
Cellulose production, activated by cyclic di-GMP through BcsA and BcsZ, is a virulence factor and an essential determinant of the three-dimensional architectures of biofilms formed by Erwinia amylovora Ea1189.Mol Plant Pathol. 2018 Jan;19(1):90-103. doi: 10.1111/mpp.12501. Epub 2016 Dec 18. Mol Plant Pathol. 2018. PMID: 27753193 Free PMC article.
-
A DIVA vaccine strain lacking RpoS and the secondary messenger c-di-GMP for protection against salmonellosis in pigs.Vet Res. 2020 Jan 10;51(1):3. doi: 10.1186/s13567-019-0730-3. Vet Res. 2020. PMID: 31924274 Free PMC article.
-
The cyclic di-GMP receptor YcgR links the second messenger with the putrescine quorum sensing system in modulation of Dickeya oryzae motility.mBio. 2025 Jul 9;16(7):e0101625. doi: 10.1128/mbio.01016-25. Epub 2025 May 30. mBio. 2025. PMID: 40444987 Free PMC article.
-
A Salmonella enterica serovar Typhimurium genome-wide CRISPRi screen reveals a role for type 1 fimbriae in evasion of antibody-mediated agglutination.Infect Immun. 2025 May 13;93(5):e0057424. doi: 10.1128/iai.00574-24. Epub 2025 Apr 10. Infect Immun. 2025. PMID: 40208041 Free PMC article.
-
Functional Regulators of Bacterial Flagella.Annu Rev Microbiol. 2019 Sep 8;73:225-246. doi: 10.1146/annurev-micro-020518-115725. Epub 2019 May 28. Annu Rev Microbiol. 2019. PMID: 31136265 Free PMC article. Review.
References
-
- Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, Benziman M. 1987. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279–281 - PubMed
-
- Povolotsky TL, Hengge R. 2012. “Life-style” control networks in Escherichia coli: signaling by the second messenger c-di-GMP. J. Biotechnol. 160:10–16 - PubMed
-
- Mills E, Pultz IS, Kulasekara HD, Miller SI. 2011. The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell. Microbiol. 13:1122–1129 - PubMed
-
- Römling U. 2012. Cyclic di-GMP, an established secondary messenger still speeding up. Environ. Microbiol. 14:1817–1829 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

