Distinct roles of highly conserved charged residues at the MotA-FliG interface in bacterial flagellar motor rotation
- PMID: 23161029
- PMCID: PMC3554000
- DOI: 10.1128/JB.01971-12
Distinct roles of highly conserved charged residues at the MotA-FliG interface in bacterial flagellar motor rotation
Abstract
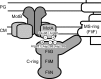
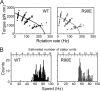
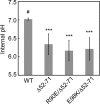
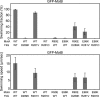
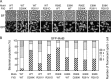
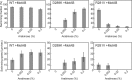
Electrostatic interactions between the stator protein MotA and the rotor protein FliG are important for bacterial flagellar motor rotation. Arg90 and Glu98 of MotA are required not only for torque generation but also for stator assembly around the rotor, but their actual roles remain unknown. Here we analyzed the roles of functionally important charged residues at the MotA-FliG interface in motor performance. About 75% of the motA(R90E) cells and 45% of the motA(E98K) cells showed no fluorescent spots of green fluorescent protein (GFP)-MotB, indicating reduced efficiency of stator assembly around the rotor. The FliG(D289K) and FliG(R281V) mutations, which restore the motility of the motA(R90E) and motA(E98K) mutants, respectively, showed reduced numbers and intensity of GFP-MotB spots as well. The FliG(D289K) mutation significantly recovered the localization of GFP-MotB to the motor in the motA(R90E) mutant, whereas the FliG(R281V) mutation did not recover the GFP-MotB localization in the motA(E98K) mutant. These results suggest that the MotA-Arg90-FliG-Asp289 interaction is critical for the proper positioning of the stators around the rotor, whereas the MotA-Glu98-FliG-Arg281 interaction is more important for torque generation.
Figures
Similar articles
-
GFP Fusion to the N-Terminus of MotB Affects the Proton Channel Activity of the Bacterial Flagellar Motor in Salmonella.Biomolecules. 2020 Aug 29;10(9):1255. doi: 10.3390/biom10091255. Biomolecules. 2020. PMID: 32872412 Free PMC article.
-
Charged residues in the cytoplasmic loop of MotA are required for stator assembly into the bacterial flagellar motor.Mol Microbiol. 2010 Dec;78(5):1117-29. doi: 10.1111/j.1365-2958.2010.07391.x. Epub 2010 Sep 27. Mol Microbiol. 2010. PMID: 21091499
-
Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli.J Bacteriol. 1999 Jun;181(11):3542-51. doi: 10.1128/JB.181.11.3542-3551.1999. J Bacteriol. 1999. PMID: 10348868 Free PMC article.
-
Structure and function of the bi-directional bacterial flagellar motor.Biomolecules. 2014 Feb 18;4(1):217-34. doi: 10.3390/biom4010217. Biomolecules. 2014. PMID: 24970213 Free PMC article. Review.
-
Scrutinizing Stator Rotation in the Bacterial Flagellum: Reconciling Experiments and Switching Models.Biomolecules. 2025 Mar 1;15(3):355. doi: 10.3390/biom15030355. Biomolecules. 2025. PMID: 40149891 Free PMC article. Review.
Cited by
-
Contribution of many charged residues at the stator-rotor interface of the Na+-driven flagellar motor to torque generation in Vibrio alginolyticus.J Bacteriol. 2014 Apr;196(7):1377-85. doi: 10.1128/JB.01392-13. Epub 2014 Jan 24. J Bacteriol. 2014. PMID: 24464458 Free PMC article.
-
Viscosity-dependent determinants of Campylobacter jejuni impacting the velocity of flagellar motility.mBio. 2024 Jan 16;15(1):e0254423. doi: 10.1128/mbio.02544-23. Epub 2023 Dec 12. mBio. 2024. PMID: 38085029 Free PMC article.
-
GFP Fusion to the N-Terminus of MotB Affects the Proton Channel Activity of the Bacterial Flagellar Motor in Salmonella.Biomolecules. 2020 Aug 29;10(9):1255. doi: 10.3390/biom10091255. Biomolecules. 2020. PMID: 32872412 Free PMC article.
-
A distant homologue of the FlgT protein interacts with MotB and FliL and is essential for flagellar rotation in Rhodobacter sphaeroides.J Bacteriol. 2013 Dec;195(23):5285-96. doi: 10.1128/JB.00760-13. Epub 2013 Sep 20. J Bacteriol. 2013. PMID: 24056105 Free PMC article.
-
ExbB cytoplasmic loop deletions cause immediate, proton motive force-independent growth arrest.J Bacteriol. 2013 Oct;195(20):4580-91. doi: 10.1128/JB.00334-13. Epub 2013 Aug 2. J Bacteriol. 2013. PMID: 23913327 Free PMC article.
References
-
- Berg HC. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 - PubMed
-
- Sowa Y, Berry RM. 2008. Bacterial flagellar motor. Q. Rev. Biophys. 41:103–132 - PubMed
-
- Braun T, Blair DF. 2001. Targeted disulfide cross-linking of the MotB protein of Escherichia coli: evidence for two H+ channels in the stator complex. Biochemistry 40:13051–13059 - PubMed
-
- Kojima S, Blair DF. 2004. Solubilization and purification of the MotA/MotB complex of Escherichia coli. Biochemistry 43:26–34 - PubMed
-
- Braun TF, Al-Mawasawi LQ, Kojima S, Blair DF. 2004. Arrangement of core membrane segments in the MotA/MotB protein-channel complex of Escherichia coli. Biochemistry 43:35–45 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

