Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development
- PMID: 23161060
- PMCID: PMC4996658
- DOI: 10.1007/s00018-012-1197-9
Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development
Abstract
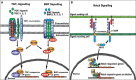
Congenital heart defects affect approximately 1-5 % of human newborns each year, and of these cardiac defects 20-30 % are due to heart valve abnormalities. Recent literature indicates that the key factors and pathways that regulate valve development are also implicated in congenital heart defects and valve disease. Currently, there are limited options for treatment of valve disease, and therefore having a better understanding of valve development can contribute critical insight into congenital valve defects and disease. There are three major signaling pathways required for early specification and initiation of endothelial-to-mesenchymal transformation (EMT) in the cardiac cushions: BMP, TGF-β, and Notch signaling. BMPs secreted from the myocardium set up the environment for the overlying endocardium to become activated; Notch signaling initiates EMT; and both BMP and TGF-β signaling synergize with Notch to promote the transition of endothelia to mesenchyme and the mesenchymal cell invasiveness. Together, these three essential signaling pathways help form the cardiac cushions and populate them with mesenchyme and, consequently, set off the cascade of events required to develop mature heart valves. Furthermore, integration and cross-talk between these pathways generate highly stratified and delicate valve leaflets and septa of the heart. Here, we discuss BMP, TGF-β, and Notch signaling pathways during mouse cardiac cushion formation and how they together produce a coordinated EMT response in the developing mouse valves.
Figures


References
-
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. - DOI - PubMed
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

