Blurry boundaries: do epithelial borderline lesions of the breast and ductal carcinoma in situ have similar rates of subsequent invasive cancer?
- PMID: 23161115
- PMCID: PMC3833840
- DOI: 10.1245/s10434-012-2719-2
Blurry boundaries: do epithelial borderline lesions of the breast and ductal carcinoma in situ have similar rates of subsequent invasive cancer?
Abstract
Background: The histology of epithelial "borderline lesions" of the breast, which have features in between atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS), is well described, but the clinical behavior is not. This study reports subsequent ipsilateral breast events (IBE) in patients with borderline lesions compared with those with DCIS.
Methods: Patients undergoing breast-conserving surgery for borderline lesions or DCIS from 1997 to 2010 were identified from a prospective database. IBE was defined as the diagnosis of subsequent ipsilateral DCIS or invasive ductal carcinoma.
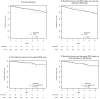
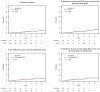
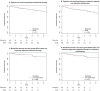
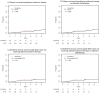
Results: A total of 143 borderline-lesion patients and 2,328 DCIS patients were identified. Median follow-up was 2.9 and 4.4 years, respectively. 7 borderline-lesion and 172 DCIS patients experienced an IBE. 5 year IBE rates were 7.7 % for borderline lesions and 7.2 % for DCIS (p = .80). 5 year invasive IBE rates were 6.5 and 2.8 %, respectively (p = .25). Similarly, when analyses were restricted to patients who did not receive radiotherapy, or endocrine therapy, or both, borderline-lesion and DCIS patients did not demonstrate statistically significant differences in rates of IBE or invasive IBE.
Conclusions: When compared with DCIS, borderline lesions do not demonstrate lower rates of IBE or invasive IBE. Despite "borderline" histology, a 5 year IBE rate of 7.7 % and an invasive IBE rate of 6.5 % suggest that the risk of future carcinoma is significant and similar to that of DCIS.
Conflict of interest statement
Figures
Similar articles
-
Comparison of ipsilateral breast tumor recurrence after breast-conserving surgery between ductal carcinoma in situ and invasive breast cancer.World J Surg Oncol. 2016 Apr 27;14:126. doi: 10.1186/s12957-016-0885-6. World J Surg Oncol. 2016. PMID: 27122132 Free PMC article.
-
Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study.J Clin Oncol. 2015 Nov 20;33(33):3938-44. doi: 10.1200/JCO.2015.60.8588. Epub 2015 Sep 14. J Clin Oncol. 2015. PMID: 26371148 Free PMC article. Clinical Trial.
-
Outcomes for Women with Minimal-Volume Ductal Carcinoma In Situ Completely Excised at Core Biopsy.Ann Surg Oncol. 2017 Dec;24(13):3888-3895. doi: 10.1245/s10434-017-6043-8. Epub 2017 Aug 21. Ann Surg Oncol. 2017. PMID: 28828599 Free PMC article.
-
Carcinoma in situ of the female breast. A clinico-pathological, immunohistological, and DNA ploidy study.APMIS Suppl. 2003;(108):1-67. APMIS Suppl. 2003. PMID: 12874968 Review.
-
Ductal carcinoma in situ of the breast: an update for the pathologist in the era of individualized risk assessment and tailored therapies.Mod Pathol. 2019 Jul;32(7):896-915. doi: 10.1038/s41379-019-0204-1. Epub 2019 Feb 13. Mod Pathol. 2019. PMID: 30760859 Review.
Cited by
-
Current management of lesions associated with an increased risk of breast cancer.Nat Rev Clin Oncol. 2015 Apr;12(4):227-38. doi: 10.1038/nrclinonc.2015.8. Epub 2015 Jan 27. Nat Rev Clin Oncol. 2015. PMID: 25622978 Review.
-
Atypical ductal or lobular hyperplasia, lobular carcinoma in-situ, flat epithelial atypia, and future risk of developing breast cancer: Systematic review and meta-analysis.Breast. 2024 Dec;78:103807. doi: 10.1016/j.breast.2024.103807. Epub 2024 Sep 11. Breast. 2024. PMID: 39270543 Free PMC article.
-
Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ.Int J Surg Pathol. 2017 Apr;25(2):100-107. doi: 10.1177/1066896916662154. Epub 2016 Aug 4. Int J Surg Pathol. 2017. PMID: 27481892 Free PMC article.
-
DEGRO practical guidelines: radiotherapy of breast cancer II: radiotherapy of non-invasive neoplasia of the breast.Strahlenther Onkol. 2014 Jan;190(1):8-16. doi: 10.1007/s00066-013-0502-3. Strahlenther Onkol. 2014. PMID: 24306068
-
Atypical ductal hyperplasia bordering on DCIS on core biopsy is associated with higher risk of upgrade than conventional atypical ductal hyperplasia.Breast Cancer Res Treat. 2020 Dec;184(3):873-880. doi: 10.1007/s10549-020-05890-1. Epub 2020 Aug 28. Breast Cancer Res Treat. 2020. PMID: 32857242 Free PMC article.
References
-
- Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–708. - PubMed
-
- Page DL. Cancer risk assessment in benign breast biopsies. Hum Pathol. 1986;17:871–4. - PubMed
-
- Page DL, Dupont WD, Rogers LW. Ductal involvement by cells of atypical lobular hyperplasia in the breast: a long-term follow-up study of cancer risk. Hum Pathol. 1988;19:201–7. - PubMed
-
- Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15:209–21. - PubMed
-
- Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23:1095–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

