Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors
- PMID: 23161173
- PMCID: PMC3887827
- DOI: 10.1007/s10059-012-0254-4
Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors
Abstract
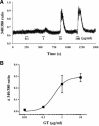
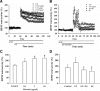
Ginseng has been shown to have memory-improving effects in human. However, little is known about the active components and the molecular mechanisms underlying its effects. Recently, we isolated novel lysophosphatidic acids (LPAs)-ginseng protein complex derived from ginseng, gintonin. Gintonin activates G protein-coupled LPA receptors with high affinity. Gintonin activated Ca²⁺-activated Clchannels in Xenopus oocytes through the activation of endogenous LPA receptor. In the present study, we investigated whether the activation of LPA receptor by gintonin is coupled to the regulation of N-methyl-D-aspartic acid (NMDA) receptor channel activity in Xenopus oocytes expressing rat NMDA receptors. The NMDA receptor-mediated ion current (I ( NMDA )) was measured using the two-electrode voltage-clamp technique. In oocytes injected with cRNAs encoding NMDA receptor subunits, gintonin enhanced I ( NMDA ) in a concentration-dependent manner. Gintonin-mediated I ( NMDA ) enhancement was blocked by Ki16425, an LPA1/3 receptor antagonist. Gintonin action was blocked by a PLC inhibitor, IP₃ receptor antagonist, Ca²⁺ chelator, and a tyrosine kinase inhibitor. The site-directed mutation of Ser1308 of the NMDA receptor, which is phosphorylated by protein kinase C (PKC), to an Ala residue, or co-expression of receptor protein tyrosine phosphatase with the NMDA receptor attenuated gintonin action. Moreover, gintonin treatment elicited a transient elevation of [Ca²⁺](i) in cultured hippocampal neurons and elevated longterm potentiation (LTP) in both concentration-dependent manners in rat hippocampal slices. Gintonin-mediated LTP induction was abolished by Ki16425. These results indicate that gintonin-mediated I ( NMDA ) potentiation and LTP induction in the hippocampus via the activation of LPA receptor might be responsible for ginseng-mediated improvement of memory-related brain functions.
Figures






Similar articles
-
Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, potentiates ATP-gated P2X₁ receptor channel currents.Mol Cells. 2013 Feb;35(2):142-50. doi: 10.1007/s10059-013-2293-x. Epub 2013 Feb 21. Mol Cells. 2013. PMID: 23456336 Free PMC article.
-
Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α.Neurosci Lett. 2013 Aug 26;548:143-8. doi: 10.1016/j.neulet.2013.05.048. Epub 2013 Jun 12. Neurosci Lett. 2013. PMID: 23769686
-
Gintonin, a novel ginseng-derived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release.Neurosci Lett. 2015 Jan 1;584:356-61. doi: 10.1016/j.neulet.2014.11.007. Epub 2014 Nov 11. Neurosci Lett. 2015. PMID: 25445364
-
Gintonin: a novel ginseng-derived ligand that targets G protein- coupled lysophosphatidic acid receptors.Curr Drug Targets. 2012 Dec;13(13):1659-64. doi: 10.2174/138945012803529947. Curr Drug Targets. 2012. PMID: 23017203 Review.
-
Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions.Front Pharmacol. 2015 Oct 27;6:245. doi: 10.3389/fphar.2015.00245. eCollection 2015. Front Pharmacol. 2015. PMID: 26578955 Free PMC article. Review.
Cited by
-
Emerging evidence that ginseng components improve cognition in subjective memory impairment, mild cognitive impairment, and early Alzheimer's disease dementia.J Ginseng Res. 2024 May;48(3):245-252. doi: 10.1016/j.jgr.2024.02.002. Epub 2024 Feb 17. J Ginseng Res. 2024. PMID: 38707644 Free PMC article. Review.
-
Korean red ginseng excitation of paraventricular nucleus neurons via non-N-methyl-D-aspartate glutamate receptor activation in mice.J Vet Sci. 2018 Mar 31;19(2):172-178. doi: 10.4142/jvs.2018.19.2.172. J Vet Sci. 2018. PMID: 29169227 Free PMC article.
-
Cognitive improvement effect of gintonin might be associated with blood-brain barrier permeability enhancement: dynamic contrast-enhanced MRI pilot study.Transl Clin Pharmacol. 2021 Mar;29(1):21-32. doi: 10.12793/tcp.2021.29.e2. Epub 2021 Mar 10. Transl Clin Pharmacol. 2021. PMID: 33854998 Free PMC article.
-
Atypical formations of gintonin lysophosphatidic acids as new materials and their beneficial effects on degenerative diseases.J Ginseng Res. 2024 Jan;48(1):1-11. doi: 10.1016/j.jgr.2023.02.004. Epub 2023 Feb 21. J Ginseng Res. 2024. PMID: 38223830 Free PMC article. Review.
-
Effects of Korean Red Ginseng extract on busulfan-induced dysfunction of the male reproductive system.J Ginseng Res. 2015 Jul;39(3):243-9. doi: 10.1016/j.jgr.2015.01.002. Epub 2015 Jan 22. J Ginseng Res. 2015. PMID: 26199556 Free PMC article.
References
-
- Carroll R.C., Zukin R.S. NMDA-receptor trafficking and targeting, implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

