Human sperm tail proteome suggests new endogenous metabolic pathways
- PMID: 23161514
- PMCID: PMC3567857
- DOI: 10.1074/mcp.M112.020552
Human sperm tail proteome suggests new endogenous metabolic pathways
Abstract
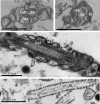
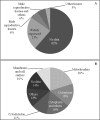
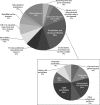
Proteomic studies are contributing greatly to our understanding of the sperm cell, and more detailed descriptions are expected to clarify additional cellular and molecular sperm attributes. The aim of this study was to characterize the subcellular proteome of the human sperm tail and, hopefully, identify less concentrated proteins (not found in whole cell proteome studies). Specifically, we were interested in characterizing the sperm metabolic proteome and gaining new insights into the sperm metabolism issue. Sperm were isolated from normozoospermic semen samples and depleted of any contaminating leukocytes. Tail fractions were obtained by means of sonication followed by sucrose-gradient ultracentrifugation, and their purity was confirmed via various techniques. Liquid chromatography and tandem mass spectrometry of isolated sperm tail peptides resulted in the identification of 1049 proteins, more than half of which had not been previously described in human sperm. The categorization of proteins according to their function revealed two main groups: proteins related to metabolism and energy production (26%), and proteins related to sperm tail structure and motility (11%). Interestingly, a great proportion of the metabolic proteome (24%) comprised enzymes involved in lipid metabolism, including enzymes for mitochondrial beta-oxidation. Unexpectedly, we also identified various peroxisomal proteins, some of which are known to be involved in the oxidation of very long chain fatty acids. Analysis of our data using Reactome suggests that both mitochondrial and peroxisomal pathways might indeed be active in sperm, and that the use of fatty acids as fuel might be more preponderant than previously thought. In addition, incubation of sperm with the fatty acid oxidation inhibitor etomoxir resulted in a significant decrease in sperm motility. Contradicting a common concept in the literature, we suggest that the male gamete might have the capacity to obtain energy from endogenous pools, and thus to adapt to putative exogenous fluctuations.
Figures

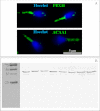
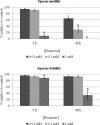
References
-
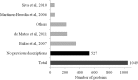
- Brewis I. A., Gadella B. M. (2010) Sperm surface proteomics: from protein lists to biological function. Mol. Hum. Reprod. 16, 68–79 - PubMed
-
- Martinez-Heredia J., Estanyol J. M., Ballesca J. L., Oliva R. (2006) Proteomic identification of human sperm proteins. Proteomics 6, 4356–4369 - PubMed
-
- Baker M. A., Reeves G., Hetherington L., Muller J., Baur I., Aitken R. J. (2007) Identification of gene products present in triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin. Appl. 1, 524–532 - PubMed
-
- de Mateo S., Martinez-Heredia J., Estanyol J. M., Dominguez-Fandos D., Vidal-Taboada J. M., Ballesca J. L., Oliva R. (2007) Marked correlations in protein expression identified by proteomic analysis of human spermatozoa. Proteomics 7, 4264–4277 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

