Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy
- PMID: 23161797
- PMCID: PMC3569655
- DOI: 10.1002/emmm.201201443
Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy
Abstract
Maintaining skeletal muscle mass is essential for general health and prevention of disease progression in various neuromuscular conditions. Currently, no treatments are available to prevent progressive loss of muscle mass in any of these conditions. Hibernating mammals are protected from muscle atrophy despite prolonged periods of immobilization and starvation. Here, we describe a mechanism underlying muscle preservation and translate it to non-hibernating mammals. Although Akt has an established role in skeletal muscle homeostasis, we find that serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates muscle mass maintenance via downregulation of proteolysis and autophagy as well as increased protein synthesis during hibernation. We demonstrate that SGK1 is critical for the maintenance of skeletal muscle homeostasis and function in non-hibernating mammals in normal and atrophic conditions such as starvation and immobilization. Our results identify a novel therapeutic target to combat loss of skeletal muscle mass associated with muscle degeneration and atrophy.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

Left column, an active summer squirrel; right column, a torpid squirrel.
The morphology of quadriceps is unchanged by hibernation as seen in haematoxylin and eosin (H&E) stained sections (scale bar 90 µm).
Dystrophin staining was performed to outline the sarcolemma to determine percentage distribution of minimum Feret's diameter.
Average ± SD of minimum Feret's diameter in quadriceps (p = 0.26) and tibialis anterior (p = 0.33) muscles is not significantly different between summer and hibernation.

Western blot of quadriceps muscle from summer active (S) and hibernating (H) squirrels using antibodies against the proteins indicated. An increased abundance of P-Foxo3a (serine-253) is accompanied by a decrease in P-Akt (serine-478). Corresponding densitometry of P-Akt and P-Foxo3a as a function of total Akt and Foxo3a.
Relative mRNA levels for four Foxo3a downstream targets show no significant changes during hibernation.
Western blots analysis of mTOR downstream targets shows significant upregulation of P-P70S6K and P-4E-BP1 during hibernation.
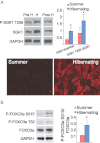
Western blot analyses and densitometry of quadriceps muscles from non-hibernating and hibernating squirrels demonstrates significant increases in total SGK1 and phosphorylated SGK1. Immunostaining of quadriceps muscle for total SGK1 shows upregulation during hibernation.
Levels of phosphorylated Foxo3a in S315 and T32 are significantly increased during hibernation. Densitometric analysis of P-Foxo3a as a function of Foxo3a.
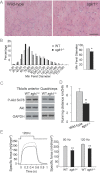
The morphology of tibialis anterior muscle as revealed by haematoxylin–eosin staining shows increased variation in muscle fiber size with numerous atrophic muscle fibers (scale bar 90 µm).
Percentage distribution and mean minimum Feret's diameter in tibialis anterior muscle (p = 0.02).
Increased levels of phosphorylated Akt (S478) in skeletal muscles of sgk1−/− mice.
sgk1−/− mice exhibit decreased running distance after 36 days of exposure to a running wheel, (p = 0.01; n = 6 animals per group).
Isometric force from soleus muscles of WT and sgk1−/− mice during repetitive electrical stimulations for 500 ms. Amplitudes of isometric force from soleus muscles at two stimulation frequencies. Specific force is plotted in all cases. Mean values ± SD from n = 9 (sgk1−/−) and n = 10 (WT) independent animals (p = 0.01).
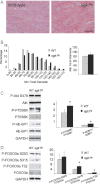
H&E staining of tibialis anterior sections from 2-month old control and transgenic mice shows no differences (scale bar 90 µm).
Morphometric analyses of minimum Feret's diameter of TA muscle reveal no changes in fiber size distribution or mean fiber size.
Western blots and densitometric analysis of muscle from transgenic and control mice. Downstream targets of the mTOR signalling cascade (p70S6K and 4EBP1) demonstrate significant upregulation of their phosphorylated forms in sgk1tg mice.
Western blot analyses and densitometry of phosphorylated FOXO3a and total FOXO3a of tibialis anterior muscles from control and transgenic mice (n = 4) show an increase of phosphorylation at S315 and T32.
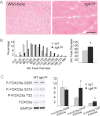
H&E staining of tibialis anterior sections reveals no changes in muscle architecture (scale bar 90 µm).
Morphometric analysis demonstrates a decrease in muscle fiber size of the wild-type control mice compared with sgk1tg transgenic littermates (p = 0.0015).
Western blot and densitometry analysis shows that levels of phosphorylated Foxo3a at S315 and T32 are increased in sgk1tg.
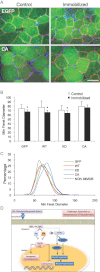
Transfection of constitutively active Sgk1 (CA) into immobilized tibialis anterior muscle (green) reveals increased fiber size diameter when compared to control, EGFP only transfected muscle fibers of immobilized tibialis anterior muscles (100 µm). Laminin γ-1 staining (red) outlines the basement membrane and blue staining marks nuclei (DAPI).
Representation of the minimum Feret's diameter average ± SD (control and immobilized; n = 9–10 per group; 800–950 fibers were measured) (*p = 0.029, *p = 0.034, *p = 0.047, p = 0.43, respectively).
Percentage distribution of the minimum Feret's diameter of tibialis anterior muscle immobilized and transfected with eGFP (GFP), wild-type Sgk1 (WT), kinase dead Sgk1 (KD) and constitutively active Sgk1 (CA) compared to non-immobilized control TA muscle (black dotted line).
Schematic representation of pathways involved in the preservation of skeletal muscle mass during prolonged periods of immobilization. SGK1, in addition to Akt, mediates protein degradation via phosphorylation of Foxo3a, with the subsequent inhibition of proteolysis and autophagy and protein synthesis through the activation of mTOR.
References
-
- Agbulut O, Vignaud A, Hourde C, Mouisel E, Fougerousse F, Butler-Browne GS, Ferry A. Slow myosin heavy chain expression in the absence of muscle activity. Am J Physiol Cell Physiol. 2009;296:C205–C214. - PubMed
-
- Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

