Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1
- PMID: 23161871
- PMCID: PMC3529370
- DOI: 10.1210/en.2012-1738
Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1
Abstract
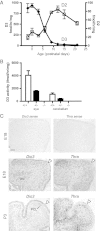
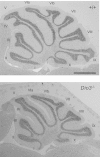
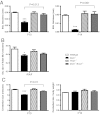
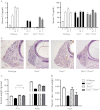
Thyroid hormone serves many functions throughout brain development, but the mechanisms that control the timing of its actions in specific brain regions are poorly understood. In the cerebellum, thyroid hormone controls formation of the transient external germinal layer, which contains proliferative granule cell precursors, subsequent granule cell migration, and cerebellar foliation. We report that the thyroid hormone-inactivating type 3 deiodinase (encoded by Dio3) is expressed in the mouse cerebellum at embryonic and neonatal stages, suggesting a need to protect cerebellar tissues from premature stimulation by thyroid hormone. Dio3(-/-) mice displayed reduced foliation, accelerated disappearance of the external germinal layer, and premature expansion of the molecular layer at juvenile ages. Furthermore, Dio3(-/-) mice exhibited locomotor behavioral abnormalities and impaired ability in descending a vertical pole. To ascertain that these phenotypes resulted from inappropriate exposure to thyroid hormone, thyroid hormone receptor α1 (TRα1) was removed from Dio3(-/-) mice, which substantially corrected the cerebellar and behavioral phenotypes. Deletion of TRα1 did not correct the previously reported small thyroid gland or deafness in Dio3(-/-) mice, indicating that Dio3 controls the activation of specific receptor isoforms in different tissues. These findings suggest that type 3 deiodinase constrains the timing of thyroid hormone action during cerebellar development.
Figures



Similar articles
-
The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function.Int J Mol Sci. 2018 Jun 19;19(6):1804. doi: 10.3390/ijms19061804. Int J Mol Sci. 2018. PMID: 29921775 Free PMC article. Review.
-
Deletion of the Thyroid Hormone-Activating Type 2 Deiodinase Rescues Cone Photoreceptor Degeneration but Not Deafness in Mice Lacking Type 3 Deiodinase.Endocrinology. 2017 Jun 1;158(6):1999-2010. doi: 10.1210/en.2017-00055. Endocrinology. 2017. PMID: 28324012 Free PMC article.
-
A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function.Endocrinology. 2009 Apr;150(4):1952-60. doi: 10.1210/en.2008-1419. Epub 2008 Dec 18. Endocrinology. 2009. PMID: 19095741 Free PMC article.
-
Regulation of mammary gland sensitivity to thyroid hormones during the transition from pregnancy to lactation.Exp Biol Med (Maywood). 2008 Oct;233(10):1309-14. doi: 10.3181/0803-RM-85. Epub 2008 Jul 18. Exp Biol Med (Maywood). 2008. PMID: 18641053
-
Genomic imprinting of the type 3 thyroid hormone deiodinase gene: regulation and developmental implications.Biochim Biophys Acta. 2013 Jul;1830(7):3946-55. doi: 10.1016/j.bbagen.2012.03.015. Epub 2012 Apr 4. Biochim Biophys Acta. 2013. PMID: 22498139 Free PMC article. Review.
Cited by
-
New insights into thyroid hormone action.Pharmacol Ther. 2017 May;173:135-145. doi: 10.1016/j.pharmthera.2017.02.012. Epub 2017 Feb 4. Pharmacol Ther. 2017. PMID: 28174093 Free PMC article. Review.
-
A marked paucity of granule cells in the developing cerebellum of the Npc1(-/-) mouse is corrected by a single injection of hydroxypropyl-β-cyclodextrin.Neurobiol Dis. 2014 Oct;70:117-26. doi: 10.1016/j.nbd.2014.06.012. Epub 2014 Jun 24. Neurobiol Dis. 2014. PMID: 24969023 Free PMC article.
-
Thyroid hormone signaling in energy homeostasis and energy metabolism.Ann N Y Acad Sci. 2014 Apr;1311:77-87. doi: 10.1111/nyas.12374. Epub 2014 Feb 20. Ann N Y Acad Sci. 2014. PMID: 24697152 Free PMC article. Review.
-
Selenium metabolism and selenoproteins function in brain and encephalopathy.Sci China Life Sci. 2025 Mar;68(3):628-656. doi: 10.1007/s11427-023-2621-7. Epub 2024 Nov 12. Sci China Life Sci. 2025. PMID: 39546178 Review.
-
The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function.Int J Mol Sci. 2018 Jun 19;19(6):1804. doi: 10.3390/ijms19061804. Int J Mol Sci. 2018. PMID: 29921775 Free PMC article. Review.
References
-
- Osler W. 1897. Sporadic cretinism in America. Transact Congr Am Physicians Surg 169–206
-
- Forrest D, Vennström B. 2000. Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

