Multiple cholinergic signaling pathways in pituitary gonadotrophs
- PMID: 23161872
- PMCID: PMC3529387
- DOI: 10.1210/en.2012-1554
Multiple cholinergic signaling pathways in pituitary gonadotrophs
Abstract
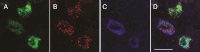
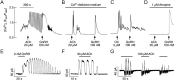
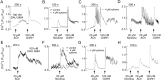
Acetylcholine (ACh) has been established as a paracrine factor in the anterior pituitary gland, but the receptors mediating ACh action and the cell types bearing these receptors have not been identified. Our results showed that the expression of the nicotinic subunits mRNAs followed the order β2 > β1 = α9 > α4 in cultured rat pituitary cells. The expression of the subunits in immortalized LβT2 mouse gonadotrophs followed the order β2 > α4 = α1. M4 > M3 muscarinic receptor mRNA were also identified in pituitary and LβT2 cells. The treatment of cultured pituitary cells with GnRH down-regulated the expression of α9 and α4 mRNAs, without affecting the expression of M3 and M4 receptor mRNAs, and ACh did not alter the expression of GnRH receptor mRNA. We also performed double immunostaining to show the expression of β2-subunit and M4 receptor proteins in gonadotrophs. Functional nicotinic channels capable of generating an inward current, facilitation of electrical activity, and Ca(2+) influx were identified in single gonadotrophs and LβT2 cells. In both cell types, the M3 receptor-mediated, phospholipase C-dependent Ca(2+) mobilization activated an outward apamin-sensitive K(+) current and caused hyperpolarization. The activation of M4 receptors by ACh inhibited cAMP production and GnRH-induced LH release in a pertussis toxin-sensitive manner. We concluded that multiple cholinergic receptors are expressed in gonadotrophs and that the main secretory action of ACh is inhibitory through M4 receptor-mediated down-regulation of cAMP production. The expression of nicotinic receptors in vitro compensates for the lack of regular GnRH stimulation of gonadotrophs.
Figures





Similar articles
-
Modulation of calcium signaling and LH secretion by progesterone in pituitary gonadotrophs and clonal pituitary cells.J Steroid Biochem Mol Biol. 1994 Jan;48(1):47-54. doi: 10.1016/0960-0760(94)90249-6. J Steroid Biochem Mol Biol. 1994. PMID: 8136305
-
Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-beta mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LbetaT2 gonadotroph cells.Mol Cell Endocrinol. 2002 Jun 28;192(1-2):55-64. doi: 10.1016/s0303-7207(02)00109-0. Mol Cell Endocrinol. 2002. PMID: 12088867
-
Stimulation of luteinizing hormone release and cyclic nucleotide production by arachidonic acid in cultured pituitary gonadotrophs.Neuroendocrinology. 1987 Jun;46(1):1-9. doi: 10.1159/000124789. Neuroendocrinology. 1987. PMID: 3039394
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
-
Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion.Front Endocrinol (Lausanne). 2017 Jun 9;8:126. doi: 10.3389/fendo.2017.00126. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28649232 Free PMC article. Review.
Cited by
-
Calcium signaling properties of a thyrotroph cell line, mouse TαT1 cells.Cell Calcium. 2015 Dec;58(6):598-605. doi: 10.1016/j.ceca.2015.09.003. Epub 2015 Sep 25. Cell Calcium. 2015. PMID: 26453278 Free PMC article.
-
Human brain imaging of nicotinic acetylcholine α4β2* receptors using [18 F]Nifene: Selectivity, functional activity, toxicity, aging effects, gender effects, and extrathalamic pathways.J Comp Neurol. 2018 Jan 1;526(1):80-95. doi: 10.1002/cne.24320. Epub 2017 Sep 19. J Comp Neurol. 2018. PMID: 28875553 Free PMC article.
-
RC-4BC cells express nicotinic and muscarinic acetylcholine receptors.PLoS One. 2022 Dec 16;17(12):e0279284. doi: 10.1371/journal.pone.0279284. eCollection 2022. PLoS One. 2022. PMID: 36525419 Free PMC article.
-
Pituitary Transcriptomic Study Reveals the Differential Regulation of lncRNAs and mRNAs Related to Prolificacy in Different FecB Genotyping Sheep.Genes (Basel). 2019 Feb 18;10(2):157. doi: 10.3390/genes10020157. Genes (Basel). 2019. PMID: 30781725 Free PMC article.
-
Distinct Expression Patterns of Osteopontin and Dentin Matrix Protein 1 Genes in Pituitary Gonadotrophs.Front Endocrinol (Lausanne). 2019 Apr 17;10:248. doi: 10.3389/fendo.2019.00248. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31057484 Free PMC article.
References
-
- Caulfield MP, Birdsall NJ. 1998. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279-290 - PubMed
-
- Hogg RC, Raggenbass M, Bertrand D. 2003. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1-46 - PubMed
-
- Zouridakis M, Zisimopoulou P, Poulas K, Tzartos SJ. 2009. Recent advances in understanding the structure of nicotinic acetylcholine receptors. IUBMB Life 61:407-423 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

