Cardiomyocyte architectural plasticity in fetal, neonatal, and adult pig hearts delineated with diffusion tensor MRI
- PMID: 23161881
- PMCID: PMC3543675
- DOI: 10.1152/ajpheart.00129.2012
Cardiomyocyte architectural plasticity in fetal, neonatal, and adult pig hearts delineated with diffusion tensor MRI
Abstract
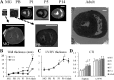
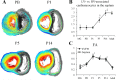
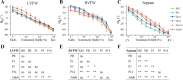
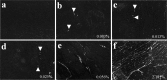
Cardiomyocyte organization is a critical determinant of coordinated cardiac contractile function. Because of the acute opening of the pulmonary circulation, the relative workload of the left ventricle (LV) and right ventricle (RV) changes substantially immediately after birth. We hypothesized that three-dimensional cardiomyocyte architecture might be required to adapt rapidly to accommodate programmed perinatal changes of cardiac function. Isolated fixed hearts from pig fetuses or pigs at midgestation, preborn, postnatal day 1 (P1), postnatal day 5, postnatal day 14 (P14), and adulthood (n = 5 for each group) were acquired for diffusion-weighted magnetic resonance imaging. Cardiomyocyte architecture was visualized by three-dimensional fiber tracking and was quantitatively evaluated by the measured helix angle (α(h)). Upon the completion of MRI, hearts were sectioned and stained with hematoxylin/eosin (H&E) to evaluate cardiomyocyte alignment, with picrosirius red to evaluate collagen content, and with anti-Ki67 to evaluate postnatal cell proliferation. The helical architecture of cardiomyocyte was observed as early as the midgestational period. Postnatal changes of cardiomyocyte architecture were observed from P1 to P14, which primary occurred in the septum and RV free wall (RVFW). In the septum, the volume ratio of LV- vs. RV-associated cardiomyocytes rapidly changed from RV-LV balanced pattern at birth to LV dominant pattern by P14. In the RVFW, subendocardial α(h) decreased by ~30° from P1 to P14. These findings indicate that the helical architecture of cardiomyocyte is developed as early as the midgestation period. Substantial and rapid adaptive changes in cardiac microarchitecture suggested considerable developmental plasticity of cardiomyocyte form and function in the postnatal period in response to altered cardiac mechanical function.
Figures



References
-
- Armour JA, Randall WC. Structural basis for cardiac function. Am J Physiol 218: 1517–1523, 1970 - PubMed
-
- Assali NS, Sehgal N, Marable S. Pulmonary and ductus arteriosus circulation in the fetal lamb before and after birth. Am J Physiol 202: 536–540, 1962 - PubMed
-
- Beinlich CJ, Rissinger CJ, Morgan HE. Mechanisms of rapid growth in the neonatal pig heart. J Mol Cell Cardiol 27: 273–281, 1995 - PubMed
-
- Bishop JE. Regulation of cardiovascular collagen deposition by mechanical forces. Mol Med Today 4: 69–75, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

