ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice
- PMID: 23161886
- PMCID: PMC3567368
- DOI: 10.1152/ajplung.00133.2012
ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice
Abstract
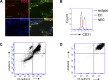
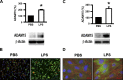
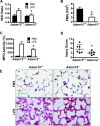
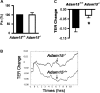
ADAM15 is a disintegrin and metalloprotease recently implicated in cancer and chronic immune disorders. We have recently characterized ADAM15 as a mediator of endothelial barrier dysfunction. Whether this molecule contributes to acute inflammation has not been evaluated. The purpose of this study was to investigate the role of ADAM15 in mediating pulmonary microvascular leakage during acute inflammatory injury. Immunofluorescent staining and Western blotting revealed that the endothelium was the main source of ADAM15 in lung tissue. In a mouse model of acute lung injury induced by lipopolysaccharide (LPS), upregulation of ADAM15 was observed in association with pulmonary edema and neutrophil infiltration. The LPS-induced inflammatory injury, as demonstrated by bronchoalveolar lavage neutrophil count, lung wet-to-dry weight ratio, and myeloperoxidase activity, was significantly attenuated in Adam15(-/-) mice. Studies with primary cell culture demonstrated abundant ADAM15 expression in endothelial cells (ECs) of mouse lung but not in neutrophils. Deficiency of ADAM15 in ECs had no obvious effect on basal permeability but significantly attenuated hyperpermeability response to LPS as evidenced by albumin flux assay and measurements of transendothelial electrical resistance, respectively. ADAM15 deficiency also reduced neutrophil chemotactic transmigration across endothelial barriers in the presence or absence of formyl-methionyl-leucyl-phenylalanine (fMLP). Rescue expression of ADAM15 in Adam15(-/-) ECs restored neutrophil transendothelial migration. These data indicate that ADAM15 upregulation contributes to inflammatory lung injury by promoting endothelial hyperpermeability and neutrophil transmigration.
Figures


References
-
- Al-Fakhri N, Wilhelm J, Hahn M, Heidt M, Hehrlein FW, Endisch AM, Hupp T, Cherian SM, Bobryshev YV, Lord RS, Katz N. Increased expression of disintegrin-metalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins alpha5beta1 and alphavbeta3 in atherosclerosis. J Cell Biochem 89: 808–823, 2003 - PubMed
-
- Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med 29: S2–S6; discussion S6–S7, 2001 - PubMed
-
- Bohm BB, Aigner T, Blobel CP, Kalden JR, Burkhardt H. Highly enhanced expression of the disintegrin metalloproteinase MDC15 (metargidin) in rheumatoid synovial tissue. Arthritis Rheum 44: 2046–2054, 2001 - PubMed
-
- Charrier L, Yan Y, Driss A, Laboisse CL, Sitaraman SV, Merlin D. ADAM-15 inhibits wound healing in human intestinal epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol 288: G346–G353, 2005 - PubMed
-
- Charrier-Hisamuddin L, Laboisse CL, Merlin D. ADAM-15: a metalloprotease that mediates inflammation. FASEB J 22: 641–653, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

