Adherence to drug-drug interaction alerts in high-risk patients: a trial of context-enhanced alerting
- PMID: 23161895
- PMCID: PMC3628050
- DOI: 10.1136/amiajnl-2012-001073
Adherence to drug-drug interaction alerts in high-risk patients: a trial of context-enhanced alerting
Abstract
Objective: Drug-drug interaction (DDI) alerting is an important form of clinical decision support, yet physicians often fail to attend to critical DDI warnings due to alert fatigue. We previously described a model for highlighting patients at high risk of a DDI by enhancing alerts with relevant laboratory data. We sought to evaluate the effect of this model on alert adherence in high-risk patients.
Methods: A 6-month randomized controlled trial involving 1029 outpatient physicians was performed. The target interactions were all DDIs known to cause hyperkalemia. Alerts in the intervention group were enhanced with the patient's most recent potassium and creatinine levels. The control group received unmodified alerts. High -risk patients were those with baseline potassium >5.0 mEq/l and/or creatinine ≥1.5 mg/dl (132 μmol/l).
Results: We found no significant difference in alert adherence in high-risk patients between the intervention group (15.3%) and the control group (16.8%) (p=0.71). Adherence in normal risk patients was significantly lower in the intervention group (14.6%) than in the control group (18.6%) (p<0.01). In neither group did physicians increase adherence in patients at high risk.
Conclusions: Physicians adhere poorly to hyperkalemia-associated DDI alerts even in patients with risk factors for a clinically significant interaction, and the display of relevant laboratory data in these alerts did not improve adherence levels in the outpatient setting. Further research is necessary to determine optimal strategies for conveying patient-specific DDI risk.
Figures

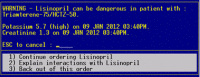
References
-
- Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 2003;163:1409–16 - PubMed
-
- Glassman PA, Simon B, Belperio P, et al. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care 2002;40:1161–71 - PubMed
-
- Hunt DL, Haynes RB, Hanna SE, et al. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA 1998;280:1339–46 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

