Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2
- PMID: 23161900
- PMCID: PMC3664379
- DOI: 10.1136/annrheumdis-2012-201978
Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2
Abstract
Objective: Notch signalling pathways are critical for angiogenesis and endothelial cell (EC) fate; however the mechanisms regulating these processes in the inflamed joint remain to be elucidated. Here, we examine whether Notch signalling mediates vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2)-induced vascular function.
Methods: Notch-1 intracellular domain (Notch-1 IC), Notch-4 IC, Delta-like-ligand 4, Hes-related transcriptional repressors-1 and 2 (Hrt-1, Hrt-2) mRNA and/or protein expression was measured by Real-time PCR and/or western blot. VEGF/Ang2 induced EC function was assessed using transwell invasion chambers, matrigel tube formation assays and wound repair scratch assays±Notch-1 siRNA or an γ-secretase inhibitor N-(N-(3,5-Difluorophenacetyl-L-alanly))-S-phenylglycine-t-Butyl Ester (DAPT) in RA synovial explants or human microvascular EC. Interleukin (IL)-6 and IL-8 were measured by ELISA and MMP2 and 9 by gelatine zymography.
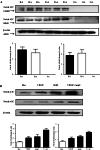
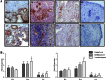
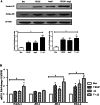
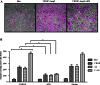
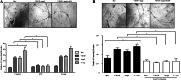
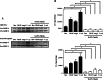
Results: Notch-1 IC and Notch-4 IC protein expressions were demonstrated in RA and psoriatic arthritis synovial biopsies, with minimal expression observed in Osteoarthritis (OA). VEGF and Ang2 induced Notch-1 IC/ Notch-4 IC protein expression in synovial explant cultures and human microvascular EC levels were further potentiated by VEGF/Ang2 stimulation in combination. Notch-1, Delta-like-ligand 4, and Hrt-2 mRNA expression were significantly induced by VEGF and Ang2 alone and in combination. Furthermore VEGF/Ang2-induced EC invasion, angiogenesis and migration were inhibited by Notch-1 siRNA or DAPT. Conditioned media from VEGF/Ang2 stimulated RA synovial explants induced EC tube formation, an effect that was inhibited by DAPT. Finally, DAPT significantly decreased VEGF/Ang2 induced IL-6, IL-8, MMP2 and 9 expressions in RA synovial explants.
Conclusions: Notch-1 mediates VEGF/Ang2-induced angiogenesis and EC invasion in inflammatory arthritis.
Keywords: Autoimmune Diseases; Inflammation; Rheumatoid Arthritis.
Figures
References
-
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996;87:1153–5 - PubMed
-
- Reece RJ, Canete JD, Parsons WJ, et al. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum 1999;42:1481–4 - PubMed
-
- Fearon U, Griosios K, Fraser A, et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol 2003;30:260–8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

