Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study
- PMID: 23161902
- PMCID: PMC3812864
- DOI: 10.1136/annrheumdis-2012-202035
Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study
Abstract
Objectives: To assess long-term golimumab efficacy/safety in patients with active psoriatic arthritis (PsA).
Methods: Adult PsA patients (≥3 swollen, ≥3 tender joints, active psoriasis) were randomly assigned to subcutaneous injections of placebo, golimumab 50 mg or 100 mg every 4 weeks (q4wks) through week 20. All patients received golimumab 50 or 100 mg beginning week 24. Findings through 2 years are reported. Efficacy evaluations included ≥20% improvement in American College of Rheumatology (ACR20) response, good/moderate response in Disease Activity Scores incorporating 28 joints and C-reactive protein (DAS28-CRP), ≥75% improvement in Psoriasis Area and Severity Index (PASI75) and changes in PsA-modified Sharp/van der Heijde scores (SHS).
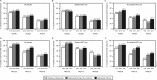
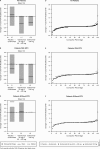
Results: Golimumab treatment through 2 years was effective in maintaining clinical response (response rates: ACR20 63%-70%, DAS28-CRP 77%-86%, PASI75 56%-72%) and inhibiting radiographic progression (mean change in PsA-modified SHS in golimumab-treated patients: -0.36), with no clear difference between doses. No new safety signals were identified through 2 years. With the study's tuberculosis screening and prophylactic measures, no patient developed active tuberculosis through 2 years.
Conclusions: Golimumab 50 and 100 mg for up to 2 years yielded sustained clinical and radiographic efficacy when administered to patients with active PsA. Increasing the golimumab dose from 50 to 100 mg q4wks added limited benefit. Golimumab safety through up to 2 years was consistent with other antitumour necrosis factor α agents used to treat PsA. Treatment of patients with latent tuberculosis identified at baseline appeared to be effective in inhibiting the development of active tuberculosis.
Keywords: Anti-TNF; Psoriatic Arthritis; Spondyloarthritis.
Figures
References
-
- Ash Z, Gaujoux-Viala C, Gossec L, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26 - PubMed
-
- Kay J, Matteson EL, Dasgupta B, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2008;58:964–75 - PubMed
-
- Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272–83 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

