Progress on first-principles-based materials design for hydrogen storage
- PMID: 23161910
- PMCID: PMC3523861
- DOI: 10.1073/pnas.1217137109
Progress on first-principles-based materials design for hydrogen storage
Abstract
This article briefly summarizes the research activities in the field of hydrogen storage in sorbent materials and reports our recent works and future directions for the design of such materials. Distinct features of sorption-based hydrogen storage methods are described compared with metal hydrides and complex chemical hydrides. We classify the studies of hydrogen sorbent materials in terms of two key technical issues: (i) constructing stable framework structures with high porosity, and (ii) increasing the binding affinity of hydrogen molecules to surfaces beyond the usual van der Waals interaction. The recent development of reticular chemistry is summarized as a means for addressing the first issue. Theoretical studies focus mainly on the second issue and can be grouped into three classes according to the underlying interaction mechanism: electrostatic interactions based on alkaline cations, Kubas interactions with open transition metals, and orbital interactions involving Ca and other nontransitional metals. Hierarchical computational methods to enable the theoretical predictions are explained, from ab initio studies to molecular dynamics simulations using force field parameters. We also discuss the actual delivery amount of stored hydrogen, which depends on the charging and discharging conditions. The usefulness and practical significance of the hydrogen spillover mechanism in increasing the storage capacity are presented as well.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
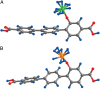
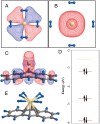
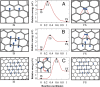
References
-
- Jacobson MZ, Delucchi MA. A path to sustainable energy by 2030. Sci Am. 2009;301(5):58–65. - PubMed
-
- George Thomas (2000) Overview of Storage Development DOE Hydrogen Program. Available at http://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/storage.pdf. Accessed October 30, 2012.
-
- van den Berg AWC, Areán CO. Materials for hydrogen storage: Current research trends and perspectives. Chem Commun (Camb) 2008;(6):668–681. - PubMed
-
- US Department of Energy (2011) DOE Targets for Onboard Hydrogen Storage Systems for Light-Duty Vehicles. Available at http://www1.eere.energy.gov/hydrogenandfuelcells/storage/current_technol.... Accessed October 30, 2012.
-
- Lim KL, Kazemian H, Yaakob Z, Daud WRW. Solid-state materials and methods for hydrogen storage: A critical review. Chem Eng Technol. 2010;33(2):213–226.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

